Improving the annual monitoring rates of testosterone replacement therapy patients in primary care
- PMID: 35914817
- PMCID: PMC9345085
- DOI: 10.1136/bmjoq-2021-001784
Improving the annual monitoring rates of testosterone replacement therapy patients in primary care
Abstract
Introduction: Testosterone replacement therapy (TRT) is the treatment of choice for male hypogonadism. British Society for Sexual Medicine (BSSM) guidelines on adult testosterone deficiency recommend that TRT patients undergo annual monitoring of their testosterone levels and potential complications of treatment; though evidence suggests that substantial numbers of men on TRT are not monitored adequately.
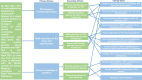
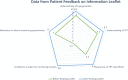
Methods: Review of the electronic patient record from a single general practice in southwest Scotland revealed that only 1 of 26 (4%) TRT patients had been monitored as per BSSM guidelines in the previous 12 months. Additionally, when monitoring was undertaken there was inconsistency in the blood tests requested. The use of quality improvement (QI) tools including process mapping and cause-and-effect diagram identified staff and patient knowledge of monitoring requirements and the lack of an effective recall system as areas for improvement. We tested three change ideas: the utilisation of an existing recall system for long-term therapies; a TRT Ordercomms blood group template (OBGT) to standardise monitoring; and a patient information leaflet (PIL) to improve patient education. The aim of this project was to achieve 60% annual monitoring rate.
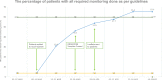
Results: The percentage of patients monitored for testosterone levels and potential TRT complications increased from 4% (1/26) to 65% (17/26) over a 7-week test period. The utilisation of the existing recall system was a particularly effective intervention, leading to an increase from 4% (1/26) to 31% (8/26) in the first 2 weeks.
Conclusion: The use of QI tools was associated with over 60% of male TRT patients receiving comprehensive annual monitoring, as per BSSM guidelines. Our findings support the hypothesis that a patient recall system, combined with an OBGT and a PIL led to this increase.
Keywords: chronic disease management; continuity of patient care; healthcare quality improvement; medication safety; patient safety.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy.BJU Int. 2009 May;103(9):1179-83. doi: 10.1111/j.1464-410X.2008.08240.x. Epub 2008 Dec 23. BJU Int. 2009. PMID: 19154450
-
Quality of Life and Sexual Function Benefits of Long-Term Testosterone Treatment: Longitudinal Results From the Registry of Hypogonadism in Men (RHYME).J Sex Med. 2017 Sep;14(9):1104-1115. doi: 10.1016/j.jsxm.2017.07.004. Epub 2017 Aug 7. J Sex Med. 2017. PMID: 28781213 Clinical Trial.
-
Adult- and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE).J Endocrinol Invest. 2022 Dec;45(12):2385-2403. doi: 10.1007/s40618-022-01859-7. Epub 2022 Aug 26. J Endocrinol Invest. 2022. PMID: 36018454 Free PMC article.
-
Testosterone Therapy: What We Have Learned From Trials.J Sex Med. 2020 Mar;17(3):447-460. doi: 10.1016/j.jsxm.2019.11.270. Epub 2020 Jan 9. J Sex Med. 2020. PMID: 31928918 Review.
-
Management of Adverse Effects in Testosterone Replacement Therapy.Int Braz J Urol. 2025 May-Jun;51(3):e20259904. doi: 10.1590/S1677-5538.IBJU.2025.9904. Int Braz J Urol. 2025. PMID: 39908204 Free PMC article. Review.
Cited by
-
Development and innovation in a new distributed medical programme: Scottish Graduate Entry Medicine (ScotGEM).Front Med (Lausanne). 2025 Jul 14;12:1586851. doi: 10.3389/fmed.2025.1586851. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40727524 Free PMC article. Review.
-
A Bibliometric Analysis of Testosterone Replacement Therapy Studies: Mapping the Scientific Landscape.Med J Islam Repub Iran. 2024 Nov 12;38:131. doi: 10.47176/mjiri.38.131. eCollection 2024. Med J Islam Repub Iran. 2024. PMID: 39968470 Free PMC article.
References
-
- King R, Peacey S. An audit of the monitoring of testosterone replacement therapy. Presented at Society for Endocrinology BES 2008, Endocrine Abstracts 15 P90, Harrogate, UK, 2008.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
