Effects of subanaesthetic S-ketamine on postoperative delirium and cognitive function in elderly patients undergoing non-cardiac thoracic surgery: a protocol for a randomised, double-blinded, placebo-controlled and positive-controlled, non-inferiority trial (SKED trial)
- PMID: 35914911
- PMCID: PMC9345033
- DOI: 10.1136/bmjopen-2022-061535
Effects of subanaesthetic S-ketamine on postoperative delirium and cognitive function in elderly patients undergoing non-cardiac thoracic surgery: a protocol for a randomised, double-blinded, placebo-controlled and positive-controlled, non-inferiority trial (SKED trial)
Abstract
Introduction: Postoperative delirium (POD) is a common and distressing complication after thoracic surgery. S-ketamine has neuroprotective properties as a dissociative anaesthetic. Emerging literature has indicated that S-ketamine can reduce cognitive impairment in patients with depression. However, the role of S-ketamine in preventing POD remains unknown. Therefore, this study aims to evaluate the effect of intraoperative prophylactic S-ketamine compared with that of dexmedetomidine on the incidence of POD in elderly patients undergoing non-cardiac thoracic surgery.
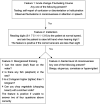
Methods and analysis: This will be a randomised, double-blinded, placebo-controlled, positive-controlled, non-inferiority trial that enrolled patients aged 60-90 years undergoing thoracic surgery. The patients will be randomly allocated in a ratio of 1:1:1 to S-ketamine, dexmedetomidine or normal saline placebo groups using computer-generated randomisation with a block size of six. The primary outcome will be the incidence of POD within 4 days after surgery and this will be assessed using a 3-Minute Diagnostic Confusion Assessment Method two times per day. The severity and duration of POD, the incidence of emergence delirium, postoperative pain, quality of sleep, cognitive function, and the plasma concentrations of acetylcholine, brain-derived neurotrophic factor, tumour necrosis factor-α and incidence of adverse events will be evaluated as secondary outcomes.
Ethics and dissemination: Ethical approval has been obtained from the Institutional Review Board of the Cancer Hospital and the Institute of Guangzhou Medical University (ZN202119). At the end of the trial, we commit to making a public disclosure available, regardless of the outcome. The public disclosure will include a publication in an appropriate journal and an oral presentation at academic meetings.
Trial registration number: ChiCTR2100052750 (NCT05242692).
Keywords: delirium & cognitive disorders; geriatric medicine; thoracic surgery.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
