Grafted hPSC-derived GABA-ergic interneurons regulate seizures and specific cognitive function in temporal lobe epilepsy
- PMID: 35915118
- PMCID: PMC9343458
- DOI: 10.1038/s41536-022-00234-7
Grafted hPSC-derived GABA-ergic interneurons regulate seizures and specific cognitive function in temporal lobe epilepsy
Abstract
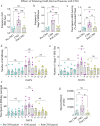
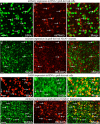
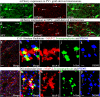
Interneuron loss/dysfunction contributes to spontaneous recurrent seizures (SRS) in chronic temporal lobe epilepsy (TLE), and interneuron grafting into the epileptic hippocampus reduces SRS and improves cognitive function. This study investigated whether graft-derived gamma-aminobutyric acid positive (GABA-ergic) interneurons directly regulate SRS and cognitive function in a rat model of chronic TLE. Human pluripotent stem cell-derived medial ganglionic eminence-like GABA-ergic progenitors, engineered to express hM4D(Gi), a designer receptor exclusively activated by designer drugs (DREADDs) through CRISPR/Cas9 technology, were grafted into hippocampi of chronically epileptic rats to facilitate the subsequent silencing of graft-derived interneurons. Such grafting substantially reduced SRS and improved hippocampus-dependent cognitive function. Remarkably, silencing of graft-derived interneurons with a designer drug increased SRS and induced location memory impairment but did not affect pattern separation function. Deactivation of DREADDs restored both SRS control and object location memory function. Thus, transplanted GABA-ergic interneurons could directly regulate SRS and specific cognitive functions in TLE.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures


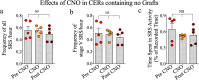

Similar articles
-
Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration.Proc Natl Acad Sci U S A. 2019 Jan 2;116(1):287-296. doi: 10.1073/pnas.1814185115. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559206 Free PMC article.
-
Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy.Stem Cells. 2010 Jul;28(7):1153-64. doi: 10.1002/stem.446. Stem Cells. 2010. PMID: 20506409 Free PMC article.
-
Neural Stem Cell or Human Induced Pluripotent Stem Cell-Derived GABA-ergic Progenitor Cell Grafting in an Animal Model of Chronic Temporal Lobe Epilepsy.Curr Protoc Stem Cell Biol. 2016 Aug 17;38:2D.7.1-2D.7.47. doi: 10.1002/cpsc.9. Curr Protoc Stem Cell Biol. 2016. PMID: 27532817 Free PMC article.
-
GABA-ergic cell therapy for epilepsy: Advances, limitations and challenges.Neurosci Biobehav Rev. 2016 Mar;62:35-47. doi: 10.1016/j.neubiorev.2015.12.014. Epub 2015 Dec 31. Neurosci Biobehav Rev. 2016. PMID: 26748379 Free PMC article. Review.
-
Potential of GABA-ergic cell therapy for schizophrenia, neuropathic pain, and Alzheimer's and Parkinson's diseases.Brain Res. 2016 May 1;1638(Pt A):74-87. doi: 10.1016/j.brainres.2015.09.019. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423935 Free PMC article. Review.
Cited by
-
Cell therapy for neurological disorders.Nat Med. 2024 Oct;30(10):2756-2770. doi: 10.1038/s41591-024-03281-3. Epub 2024 Oct 15. Nat Med. 2024. PMID: 39407034 Review.
-
Cell-specific extracellular vesicle-encapsulated exogenous GABA controls seizures in epilepsy.Stem Cell Res Ther. 2024 Apr 19;15(1):108. doi: 10.1186/s13287-024-03721-4. Stem Cell Res Ther. 2024. PMID: 38637847 Free PMC article.
-
Masterminding Hippocampal Circuits by Transplanting Human GABAergic Interneurons to Treat Temporal Lobe Epilepsy.Epilepsy Curr. 2023 Jun 21;23(4):268-270. doi: 10.1177/15357597231175010. eCollection 2023 Jul-Aug. Epilepsy Curr. 2023. PMID: 37662454 Free PMC article. No abstract available.
-
Stem Cells: Recent Developments Redefining Epilepsy Therapy.Cell Transplant. 2023 Jan-Dec;32:9636897231158967. doi: 10.1177/09636897231158967. Cell Transplant. 2023. PMID: 36919673 Free PMC article. Review.
-
Geraniol Ameliorates Pentylenetetrazol-Induced Epilepsy, Neuroinflammation, and Oxidative Stress via Modulating the GABAergic Tract: In vitro and in vivo studies.Drug Des Devel Ther. 2024 Dec 5;18:5655-5672. doi: 10.2147/DDDT.S481985. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39654600 Free PMC article.
References
Grants and funding
- IK6 BX003612/BX/BLRD VA/United States
- I01 BX000883/BX/BLRD VA/United States
- R24 NS086604/NS/NINDS NIH HHS/United States
- P50 MH100031/MH/NIMH NIH HHS/United States
- R01 MH100031/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- W81XWH-14-1-0558/U.S. Department of Defense (United States Department of Defense)
- R01 NS096282/NS/NINDS NIH HHS/United States
- R01 NS106907/NS/NINDS NIH HHS/United States
- R01 NS086604/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- I01BX000883/U.S. Department of Veterans Affairs (Department of Veterans Affairs)
LinkOut - more resources
Full Text Sources
Research Materials

