The E3 ubiquitin ligase HECTD1 contributes to cell proliferation through an effect on mitosis
- PMID: 35915203
- PMCID: PMC9343455
- DOI: 10.1038/s41598-022-16965-y
The E3 ubiquitin ligase HECTD1 contributes to cell proliferation through an effect on mitosis
Abstract
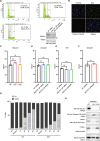
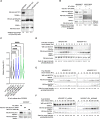
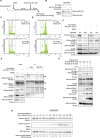
The cell cycle is tightly regulated by protein phosphorylation and ubiquitylation events. During mitosis, the multi-subunit cullin-RING E3 ubiquitin ligase APC/c functions as a molecular switch which signals for one cell to divide into two daughter cells, through the ubiquitylation and proteasomal degradation of mitotic cyclins. The contributions of other E3 ligase families during cell cycle progression remain less well understood. Similarly, the roles of ubiquitin chain types beyond homotypic K48 chains in S-phase or branched K11/K48 chains during mitosis, also remain to be fully determined. Our recent findings that HECTD1 ubiquitin ligase activity assembles branched K29/K48 ubiquitin linkages prompted us to evaluate HECTD1 function during the cell cycle. We used transient knockdown and genetic knockout to show that HECTD1 depletion in HEK293T and HeLa cells decreases cell number and we established that this is mediated through loss of ubiquitin ligase activity. Interestingly, we found that HECTD1 depletion increases the proportion of cells with aligned chromosomes (Prometa/Metaphase) and we confirmed this molecularly using phospho-Histone H3 (Ser28) as a marker of mitosis. Time-lapse microscopy of NEBD to anaphase onset established that HECTD1-depleted cells take on average longer to go through mitosis. In line with this data, HECTD1 depletion reduced the activity of the Spindle Assembly Checkpoint, and BUB3, a component of the Mitosis Checkpoint Complex, was identified as novel HECTD1 interactor. BUB3, BUBR1 or MAD2 protein levels remained unchanged in HECTD1-depleted cells. Overall, this study reveals a novel putative role for HECTD1 during mitosis and warrants further work to elucidate the mechanisms involved.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The deubiquitinase TRABID stabilizes the K29/K48-specific E3 ubiquitin ligase HECTD1.J Biol Chem. 2021 Jan-Jun;296:100246. doi: 10.1074/jbc.RA120.015162. Epub 2021 Jan 8. J Biol Chem. 2021. PMID: 33853758 Free PMC article.
-
Role of ubiquitylation of components of mitotic checkpoint complex in their dissociation from anaphase-promoting complex/cyclosome.Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):1777-1782. doi: 10.1073/pnas.1720312115. Epub 2018 Feb 5. Proc Natl Acad Sci U S A. 2018. PMID: 29432156 Free PMC article.
-
Role of ubiquitin-protein ligase UBR5 in the disassembly of mitotic checkpoint complexes.Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2121478119. doi: 10.1073/pnas.2121478119. Proc Natl Acad Sci U S A. 2022. PMID: 35217622 Free PMC article.
-
Impressionist portraits of mitotic exit: APC/C, K11-linked ubiquitin chains and Cezanne.Cell Cycle. 2019 Mar-Apr;18(6-7):652-660. doi: 10.1080/15384101.2019.1593646. Epub 2019 Mar 28. Cell Cycle. 2019. PMID: 30874463 Free PMC article. Review.
-
Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis.Biochim Biophys Acta. 2014 Apr;1845(2):277-93. doi: 10.1016/j.bbcan.2014.02.001. Epub 2014 Feb 22. Biochim Biophys Acta. 2014. PMID: 24569229 Free PMC article. Review.
Cited by
-
Centriolin interacts with HectD1 in a cell cycle dependent manner.BMC Res Notes. 2023 Dec 19;16(1):375. doi: 10.1186/s13104-023-06670-y. BMC Res Notes. 2023. PMID: 38115153 Free PMC article.
-
A Brief Overview of the Epigenetic Regulatory Mechanisms in Plants.Int J Mol Sci. 2025 May 14;26(10):4700. doi: 10.3390/ijms26104700. Int J Mol Sci. 2025. PMID: 40429841 Free PMC article. Review.
-
Trabid patient mutations impede the axonal trafficking of adenomatous polyposis coli to disrupt neurite growth.Elife. 2023 Dec 15;12:RP90796. doi: 10.7554/eLife.90796. Elife. 2023. PMID: 38099646 Free PMC article.
-
Sequence diversity lost in early pregnancy.Nature. 2025 Jun;642(8068):672-681. doi: 10.1038/s41586-025-09031-w. Epub 2025 May 21. Nature. 2025. PMID: 40399685 Free PMC article.
-
Genome-wide association and transcriptomic analysis and the identification of growth-related genes in Macrobrachium nipponense.BMC Genomics. 2024 Dec 5;25(1):1182. doi: 10.1186/s12864-024-11105-4. BMC Genomics. 2024. PMID: 39639210 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

