Detection tools for prediction and identification of adverse drug reactions in older patients: a systematic review and meta-analysis
- PMID: 35915219
- PMCID: PMC9341414
- DOI: 10.1038/s41598-022-17410-w
Detection tools for prediction and identification of adverse drug reactions in older patients: a systematic review and meta-analysis
Abstract
Tools to accurately predict and detect adverse drug reactions (ADR) in elderly patients have not been developed. We aimed to identify and evaluate reports on tools that predict and detect ADR in elderly patients (≥ 60 years). In this review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Databases were searched until January 2022 using key terms "elderly," "adverse drug reaction," and "detection instruments." Eighteen studies met the inclusion criteria, and they examined assorted interventions: STOPP/START version 1/2 (n = 10), Beers Criteria 2012 or 2015 (n = 4), Systematic Tool to Reduce Inappropriate Prescribing (STRIP) (n = 2), Tool to Reduce Inappropriate Medications (TRIM) (n = 1), Medication Risk Score (MERIS) (n = 1), Computerized alert systems (n = 1), and Norwegian General Practice-Nursing Home criteria (n = 1). The interventions affected the number of potential prescription omissions (OR, 0.50 [0.37-0.69]; p < 0.0001; four studies). No apparent reduction in the number of drug interactions within 2 months (OR, 0.84 [0.70-1.02]; p = 0.08; two studies) and mortality (OR, 0.92 [0.76-1.12]; p = 0.41; three studies) was observed. In conclusion, there is no definitive and validated assessment tool for detecting and predicting ADR in elderly patients. Thus, more research on refining existing tools or developing new ones is warranted.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

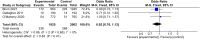
Similar articles
-
The Association Between Potentially Inappropriate Prescribing and Medication-Related Hospital Admissions in Older Patients: A Nested Case Control Study.Drug Saf. 2016 Jan;39(1):79-87. doi: 10.1007/s40264-015-0361-1. Drug Saf. 2016. PMID: 26553305
-
Potentially inappropriate prescribing in an Irish elderly population in primary care.Br J Clin Pharmacol. 2009 Dec;68(6):936-47. doi: 10.1111/j.1365-2125.2009.03531.x. Br J Clin Pharmacol. 2009. PMID: 20002089 Free PMC article.
-
Potentially inappropriate medications in a sample of Portuguese nursing home residents: Does the choice of screening tools matter?Int J Clin Pharm. 2016 Oct;38(5):1103-11. doi: 10.1007/s11096-016-0337-y. Epub 2016 Jun 24. Int J Clin Pharm. 2016. PMID: 27343120
-
STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: origin and progress.Expert Rev Clin Pharmacol. 2020 Jan;13(1):15-22. doi: 10.1080/17512433.2020.1697676. Epub 2019 Nov 30. Expert Rev Clin Pharmacol. 2020. PMID: 31790317 Review.
-
Reducing Potentially Inappropriate Prescriptions for Older Patients Using Computerized Decision Support Tools: Systematic Review.J Med Internet Res. 2019 Nov 14;21(11):e15385. doi: 10.2196/15385. J Med Internet Res. 2019. PMID: 31724956 Free PMC article.
Cited by
-
Prevalence of Potentially Inappropriate Prescriptions According to the New STOPP/START Criteria in Nursing Homes: A Systematic Review.Healthcare (Basel). 2023 Feb 1;11(3):422. doi: 10.3390/healthcare11030422. Healthcare (Basel). 2023. PMID: 36766997 Free PMC article. Review.
-
Characteristics of isoniazid-induced psychosis: a systematic review of case reports and case series.Eur J Clin Pharmacol. 2024 Nov;80(11):1725-1740. doi: 10.1007/s00228-024-03738-x. Epub 2024 Aug 13. Eur J Clin Pharmacol. 2024. PMID: 39134879 Free PMC article.
-
Comparing prevalence and types of potentially inappropriate medications among patient groups in a post-acute and secondary care hospital.Sci Rep. 2023 Sep 15;13(1):14543. doi: 10.1038/s41598-023-41617-0. Sci Rep. 2023. PMID: 37714927 Free PMC article.
-
Potentially Inappropriate Prescribing and Potential Prescribing Omissions and Their Association with Adverse Drug Reaction-Related Hospital Admissions.J Clin Med. 2024 Jan 6;13(2):323. doi: 10.3390/jcm13020323. J Clin Med. 2024. PMID: 38256457 Free PMC article.
-
Methods for identifying adverse drug reactions in primary care: A systematic review.PLoS One. 2025 Feb 4;20(2):e0317660. doi: 10.1371/journal.pone.0317660. eCollection 2025. PLoS One. 2025. PMID: 39903764 Free PMC article.
References
-
- (EMA) EMA. Guideline on good pharmacovigilance practices (GVP). Definition. 2017(United Kingdom): Annex I.
-
- Food and Drug Administration Department of Health and Human Services, Chapter I. United States and America. Subchapter D—Drugs for human use. FDA. In: FDA; 2018.
-
- Barclay K, Frasetto A, Robb J, Mandel ED. Polypharmacy in the elderly: How to reduce adverse drug events. Clin. Rev. 2018;28:38–44.
-
- Andersen LV, Poulsen BK, Poulsen MH, Krogh ML. The ESC Handbook on Cardiovascular Pharmacotherapy. 2. Oxford University Press; 2019. Major drug interactions; pp. 387–410.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

