Detection tools for prediction and identification of adverse drug reactions in older patients: a systematic review and meta-analysis
- PMID: 35915219
- PMCID: PMC9341414
- DOI: 10.1038/s41598-022-17410-w
Detection tools for prediction and identification of adverse drug reactions in older patients: a systematic review and meta-analysis
Abstract
Tools to accurately predict and detect adverse drug reactions (ADR) in elderly patients have not been developed. We aimed to identify and evaluate reports on tools that predict and detect ADR in elderly patients (≥ 60 years). In this review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Databases were searched until January 2022 using key terms "elderly," "adverse drug reaction," and "detection instruments." Eighteen studies met the inclusion criteria, and they examined assorted interventions: STOPP/START version 1/2 (n = 10), Beers Criteria 2012 or 2015 (n = 4), Systematic Tool to Reduce Inappropriate Prescribing (STRIP) (n = 2), Tool to Reduce Inappropriate Medications (TRIM) (n = 1), Medication Risk Score (MERIS) (n = 1), Computerized alert systems (n = 1), and Norwegian General Practice-Nursing Home criteria (n = 1). The interventions affected the number of potential prescription omissions (OR, 0.50 [0.37-0.69]; p < 0.0001; four studies). No apparent reduction in the number of drug interactions within 2 months (OR, 0.84 [0.70-1.02]; p = 0.08; two studies) and mortality (OR, 0.92 [0.76-1.12]; p = 0.41; three studies) was observed. In conclusion, there is no definitive and validated assessment tool for detecting and predicting ADR in elderly patients. Thus, more research on refining existing tools or developing new ones is warranted.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
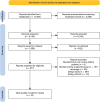
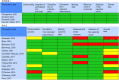

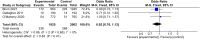
References
-
- (EMA) EMA. Guideline on good pharmacovigilance practices (GVP). Definition. 2017(United Kingdom): Annex I.
-
- Food and Drug Administration Department of Health and Human Services, Chapter I. United States and America. Subchapter D—Drugs for human use. FDA. In: FDA; 2018.
-
- Barclay K, Frasetto A, Robb J, Mandel ED. Polypharmacy in the elderly: How to reduce adverse drug events. Clin. Rev. 2018;28:38–44.
-
- Andersen LV, Poulsen BK, Poulsen MH, Krogh ML. The ESC Handbook on Cardiovascular Pharmacotherapy. 2. Oxford University Press; 2019. Major drug interactions; pp. 387–410.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

