Improved Aerosolization Stability of Inhalable Tobramycin Powder Formulation by Co-Spray Drying with Colistin
- PMID: 35915320
- PMCID: PMC10019100
- DOI: 10.1007/s11095-022-03344-5
Improved Aerosolization Stability of Inhalable Tobramycin Powder Formulation by Co-Spray Drying with Colistin
Abstract
Purpose: Tobramycin shows synergistic antibacterial activity with colistin and can reduce the toxic effects of colistin. The purpose of this study is to prepare pulmonary powder formulations containing both colistin and tobramycin and to assess their in vitro aerosol performance and storage stability.
Methods: The dry powder formulations were manufactured using a lab-scale spray dryer. In vitro aerosol performance was measured using a Next Generation Impactor. The storage stability of the dry powder formulations was measured at 22°C and two relative humidity levels - 20 and 55%. Colistin composition on the particle surface was measured using X-ray photoelectron spectroscopy.
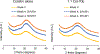
Results: Two combination formulations, with 1:1 and 1:5 molar ratios of colistin and tobramycin, showed fine particle fractions (FPF) of 85%, which was significantly higher than that of the spray dried tobramycin (45%). FPF of the tobramycin formulation increased significantly when stored for four weeks at both 20% and 55% RH. In contrast, FPF values of both combination formulations and spray dried colistin remained stable at both humidity levels. Particle surface of each combination was significantly enriched in colistin molecules; 1:5 combination showed 77% by wt. colistin.
Conclusions: The superior aerosol performance and aerosolization stability of 1:1 and 1:5 combination formulations of colistin and tobramycin could be attributed to enrichment of colistin on the co-spray dried particle surface. The observed powder properties may be the result of a surfactant-like assembly of these colistin molecules during spray drying, thus forming a hydrophobic particle surface.
Keywords: combination antibiotics; dry powder inhalation; respiratory infections; storage stability.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
CONFLICT OF INTEREST
MUA, MAKA, JL and QTZ are inventors of PCT/US2021/030393.
Figures









References
-
- Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55(7):3284–94. 10.1128/Aac.01733-10. - DOI - PMC - PubMed
-
- Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob Agents Chemother. 2014;58(5):2570–9. 10.1128/aac.01705-13. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

