Impact of rapid molecular testing on diagnosis, treatment and management of community-acquired pneumonia in Norway: a pragmatic randomised controlled trial (CAPNOR)
- PMID: 35915452
- PMCID: PMC9340738
- DOI: 10.1186/s13063-022-06467-7
Impact of rapid molecular testing on diagnosis, treatment and management of community-acquired pneumonia in Norway: a pragmatic randomised controlled trial (CAPNOR)
Abstract
Background: Community-acquired pneumonia (CAP) causes a large burden of disease. Due to difficulties in obtaining representative respiratory samples and insensitive standard microbiological methods, the microbiological aetiology of CAP is difficult to ascertain. With a few exceptions, standard-of-care diagnostics are too slow to influence initial decisions on antimicrobial therapy. The management of CAP is therefore largely based on empirical treatment guidelines. Empiric antimicrobial therapy is often initiated in the primary care setting, affecting diagnostic tests based on conventional bacterial culture in hospitalized patients. Implementing rapid molecular testing may improve both the proportion of positive tests and the time it takes to obtain test results. Both measures are important for initiation of pathogen-targeted antibiotics, involving rapid de-escalation or escalation of treatment, which may improve antimicrobial stewardship and potentially patient outcome.
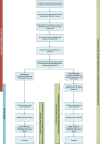
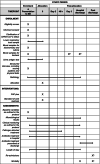
Methods: Patients presenting to the emergency department of Haukeland University Hospital (HUH) in Bergen, Norway, will be screened for inclusion into a pragmatic randomised controlled trial (RCT). Eligible patients with a suspicion of CAP will be included and randomised to receive either standard-of-care methods (standard microbiological testing) or standard-of-care methods in addition to testing by the rapid and comprehensive real-time multiplex PCR panel, the BioFire® FilmArray® Pneumonia Panel plus (FAP plus) (bioMérieux S.A., Marcy-l'Etoile, France). The results of the FAP plus will be communicated directly to the treating staff within ~2 h of sampling.
Discussion: We will examine if rapid use of FAP plus panel in hospitalized patients with suspected CAP can improve both the time to and the proportion of patients receiving pathogen-directed treatment, thereby shortening the exposure to unnecessary antibiotics and the length of hospital admission, compared to the standard-of-care arm. The pragmatic design together with broad inclusion criteria and a straightforward intervention could make our results generalizable to other similar centres.
Trial registration: ClinicalTrials.gov NCT04660084 . Registered on December 9, 2020.
Keywords: Antimicrobial treatment; Community-acquired pneumonia; FilmArray Pneumonia Panel; Induced sputum; Microbiological testing; Molecular testing; Rapid diagnostics; Respiratory tract infections; Syndromic PCR panel.
© 2022. The Author(s).
Conflict of interest statement
TWC has received speaker fees, honoraria, travel reimbursement and equipment and consumables at a discount or free of charge for the purposes independent of research, outside of this submitted study, from BioFire diagnostics, BioMérieux and QIAGEN. TWC has received consultancy fees from Cepheid, Synairgen research, Randox laboratories and Cidara therapeutics. He has received honoraria for participation in advisory boards and consultancy fees from Cepheid, Roche, Janssen and Shionogi. He is a member of independent data monitoring committees for trials sponsored by Roche. He has acted as the UK chief investigator for studies sponsored by Janssen. C. H. van Werkhoven has received non-financial and financial research support from BioMérieux outside this submitted study. The other authors have nothing to disclose.
Figures
References
-
- Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

