Differences in epidemiology of enteropathogens in children pre- and post-rotavirus vaccine introduction in Kilifi, coastal Kenya
- PMID: 35915480
- PMCID: PMC9340678
- DOI: 10.1186/s13099-022-00506-z
Differences in epidemiology of enteropathogens in children pre- and post-rotavirus vaccine introduction in Kilifi, coastal Kenya
Abstract
Background: Kenya introduced Rotarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium) vaccination into its national immunization programme beginning July 2014. The impact of this vaccination program on the local epidemiology of various known enteropathogens is not fully understood.
Methods: We used a custom TaqMan Array Card (TAC) to screen for 28 different enteropathogens in 718 stools from children aged less than 13 years admitted to Kilifi County Hospital, coastal Kenya, following presentation with diarrhea in 2013 (before vaccine introduction) and in 2016-2018 (after vaccine introduction). Pathogen positivity rate differences between pre- and post-Rotarix® vaccination introduction were examined using both univariate and multivariable logistic regression models.
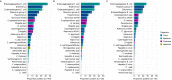
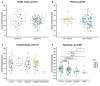
Results: In 665 specimens (92.6%), one or more enteropathogen was detected, while in 323 specimens (48.6%) three or more enteropathogens were detected. The top six detected enteropathogens were: enteroaggregative Escherichia coli (EAggEC; 42.1%), enteropathogenic Escherichia coli (EPEC; 30.2%), enterovirus (26.9%), rotavirus group A (RVA; 24.8%), parechovirus (16.6%) and norovirus GI/GII (14.4%). Post-rotavirus vaccine introduction, there was a significant increase in the proportion of samples testing positive for EAggEC (35.7% vs. 45.3%, p = 0.014), cytomegalovirus (4.2% vs. 9.9%, p = 0.008), Vibrio cholerae (0.0% vs. 2.3%, p = 0.019), Strongyloides species (0.8% vs. 3.6%, p = 0.048) and Dientamoeba fragilis (2.1% vs. 7.8%, p = 0.004). Although not reaching statistical significance, the positivity rate of adenovirus 40/41 (5.8% vs. 7.3%, p = 0.444), norovirus GI/GII (11.2% vs. 15.9%, p = 0.089), Shigella species (8.7% vs. 13.0%, p = 0.092) and Cryptosporidium spp. (11.6% vs. 14.7%, p = 0.261) appeared to increase post-vaccine introduction. Conversely, the positivity rate of sapovirus decreased significantly post-vaccine introduction (7.8% vs. 4.0%, p = 0.030) while that of RVA appeared not to change (27.4% vs. 23.5%, p = 0.253). More enteropathogen coinfections were detected per child post-vaccine introduction compared to before (mean: 2.7 vs. 2.3; p = 0.0025).
Conclusions: In this rural Coastal Kenya setting, childhood enteropathogen infection burden was high both pre- and post-rotavirus vaccination introduction. Children who had diarrheal admissions post-vaccination showed an increase in coinfections and changes in specific enteropathogen positivity rates. This study highlights the utility of multipathogen detection platforms such as TAC in understanding etiology of childhood acute gastroenteritis in resource-limited regions.
Keywords: Children; Co-infections; Enteropathogens; Epidemiology; Kenya; RVA; Taqman array card; Vaccination.
© 2022. The Author(s).
Conflict of interest statement
All the authors declare that they have no competing interests.
Figures

References
-
- Troeger C, Blacker BF, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases. 2018;18:1211–28. doi: 10.1016/S1473-3099(18)30362-1. - DOI - PMC - PubMed
-
- Rogawski ET, Liu J, Platts-Mills JA, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018;6:e1319-e28. doi: 10.1016/S2214-109X(18)30351-6. - DOI - PMC - PubMed
-
- WHO Rotavirus vaccines: WHO position paper- July 2021. Weekly Epidemiological Record. 2021;96:301–219.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

