Effectiveness of the 10-valent pneumococcal conjugate vaccine on pediatric pneumonia confirmed by ultrasound: a matched case-control study
- PMID: 35915495
- PMCID: PMC9341060
- DOI: 10.1186/s12931-022-02115-5
Effectiveness of the 10-valent pneumococcal conjugate vaccine on pediatric pneumonia confirmed by ultrasound: a matched case-control study
Abstract
Background: Bangladesh introduced the 10-valent pneumococcal conjugate vaccine (PCV10) for children aged < 1 year in March 2015. Previous vaccine effectiveness (VE) studies for pneumonia have used invasive pneumococcal disease or chest X-rays. None have used ultrasound. We sought to determine the VE of PCV10 against sonographically-confirmed pneumonia in three subdistrict health complexes in Bangladesh.
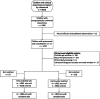
Methods: We conducted a matched case-control study between July 2015 and September 2017 in three subdistricts of Sylhet, Bangladesh. Cases were vaccine-eligible children aged 3-35 months with sonographically-confirmed pneumonia, who were matched with two types of controls by age, sex, week of diagnosis, subdistrict health complex (clinic controls) or distance from subdistrict health complex (community controls) and had an illness unlikely due to Streptococcus pneumoniae (clinic controls) or were healthy (community controls). VE was measured using multivariable conditional logistic regression.
Results: We evaluated 8926 children (average age 13.3 months, 58% boys) with clinical pneumonia by ultrasound; 2470 had pneumonia with consolidations ≥ 1 cm; 1893 pneumonia cases were matched with 4238 clinic controls; and 1832 were matched with 3636 community controls. VE increased with the threshold used for consolidation size on ultrasound: the adjusted VE of ≥ 2 doses vs. non-recipients of PCV10 against pneumonia increased from 15.8% (95% CI 1.6-28.0%) for consolidations ≥ 1 cm to 29.6% (12.8-43.2%) for consolidations ≥ 1.5 cm using clinic controls and from 2.7% (- 14.2-17.2%) to 23.5% (4.4-38.8%) using community controls, respectively.
Conclusions: PCV10 was effective at reducing sonographically-confirmed pneumonia in children aged 3-35 months of age when compared to unvaccinated children. VE increased with the threshold used for consolidation size on ultrasound in clinic and community controls alike. This study provides evidence that lung ultrasound is a useful alternative to chest X-ray for case-control studies evaluating the effectiveness of vaccines against pneumonia.
Keywords: Child health; Pneumococcal conjugate vaccine; Pneumonia; South Asia; Ultrasound; Vaccine effectiveness.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




References
-
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. - DOI - PMC - PubMed
-
- Wahl B, O'Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Lukšić I, Nair H, McAllister DA, Campbell H, Rudan I, Black R, Knoll MD. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–e757. doi: 10.1016/S2214-109X(18)30247-X. - DOI - PMC - PubMed
-
- Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, McAdam KP, Biney E, Saaka M, Onwuchekwa U, Yallop F, Pierce NF, Greenwood BM, Adegbola RA. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

