The ethanol inhibition of basolateral amygdala neuron spiking is mediated by a γ-aminobutyric acid type A-mediated tonic current
- PMID: 35915568
- PMCID: PMC9509443
- DOI: 10.1111/acer.14918
The ethanol inhibition of basolateral amygdala neuron spiking is mediated by a γ-aminobutyric acid type A-mediated tonic current
Abstract
Background: The basolateral nucleus of the amygdala (BLA) plays an important role in the development of fear and anxiety-related behaviors. The BLA receives inputs from all sensory stimuli. After processing those stimuli, BLA neurons signal neurons within the central amygdala and other brain regions, including the ventral and dorsal striatum and frontal cortex. Studies suggest that the BLA is involved in drug dependence and in the reinforcing actions of ethanol. For example, acute exposure to ethanol reduces anxiety, while withdrawal from chronic ethanol exposure alters BLA synaptic transmission, which increases anxiety, a common underlying cause of relapse. Exposure to and withdrawal from chronic alcohol also disrupts many brain areas that connect with the BLA. Despite these important findings, the acute actions of alcohol on the intrinsic excitability of BLA neurons have not been fully characterized.
Methods: Brain slices containing the BLA were prepared from adult C57BL/6J male mice. Whole-cell and sharp electrode electrophysiological recordings were performed to characterize the effects of acute ethanol on BLA neuronal and astrocyte function, respectively.
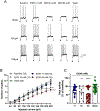
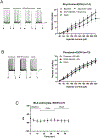
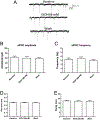
Results: Ethanol inhibited action potential (AP) firing of BLA neurons but had no effect on BLA astrocyte resting membrane potential. The ethanol-induced inhibition of firing was concentration-dependent (11 to 66 mM) and accompanied by a reduction in the input resistance and an increase in the rheobase of BLA neurons. The inhibitory effect of ethanol was suppressed by picrotoxin, which blocks both γ-aminobutyric acid type A (GABAA ) and glycine receptors, but not by the selective glycine receptor antagonist strychnine, which suggests an involvement of GABAA receptors. Ethanol did not affect spontaneous inhibitory postsynaptic currents suggesting that the inhibition of BLA neuronal excitability by ethanol was not due to an increase in GABAA -mediated synaptic transmission. However, acute ethanol enhanced the amplitude of the holding current of BLA neurons, an effect that was prevented by picrotoxin, which by itself reduced the holding current.
Conclusions: These results suggest that BLA neurons express a GABA-mediated tonic current that is enhanced by acute ethanol, which leads to reduced excitability of BLA neurons.
Keywords: GABAA-mediated tonic currents; basolateral amygdala; ethanol; intrinsic excitability.
© 2022 Research Society on Alcoholism.
Figures
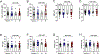
Similar articles
-
Chronic intermittent ethanol exposure differentially alters the excitability of neurons in the orbitofrontal cortex and basolateral amygdala that project to the dorsal striatum.Neuropharmacology. 2023 May 1;228:109463. doi: 10.1016/j.neuropharm.2023.109463. Epub 2023 Feb 13. Neuropharmacology. 2023. PMID: 36792030 Free PMC article.
-
Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism.Neuropsychopharmacology. 2013 Jun;38(7):1176-88. doi: 10.1038/npp.2013.12. Epub 2013 Jan 11. Neuropsychopharmacology. 2013. PMID: 23314219 Free PMC article.
-
Acute Ethanol Modulates Synaptic Inhibition in the Basolateral Amygdala via Rapid NLRP3 Inflammasome Activation and Regulates Anxiety-Like Behavior in Rats.J Neurosci. 2023 Nov 22;43(47):7902-7912. doi: 10.1523/JNEUROSCI.1744-22.2023. J Neurosci. 2023. PMID: 37739795 Free PMC article.
-
The basolateral amygdala γ-aminobutyric acidergic system in health and disease.J Neurosci Res. 2016 Jun;94(6):548-67. doi: 10.1002/jnr.23690. Epub 2015 Nov 19. J Neurosci Res. 2016. PMID: 26586374 Free PMC article. Review.
-
Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies.Alcohol. 2007 May;41(3):187-99. doi: 10.1016/j.alcohol.2007.04.004. Epub 2007 May 23. Alcohol. 2007. PMID: 17521847 Free PMC article. Review.
Cited by
-
Chronic intermittent ethanol exposure differentially alters the excitability of neurons in the orbitofrontal cortex and basolateral amygdala that project to the dorsal striatum.Neuropharmacology. 2023 May 1;228:109463. doi: 10.1016/j.neuropharm.2023.109463. Epub 2023 Feb 13. Neuropharmacology. 2023. PMID: 36792030 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

