Enhanced anaerobic digestion of dairy wastewater in a granular activated carbon amended sequential batch reactor
- PMID: 35915605
- PMCID: PMC9324911
- DOI: 10.1111/gcbb.12947
Enhanced anaerobic digestion of dairy wastewater in a granular activated carbon amended sequential batch reactor
Abstract
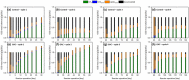
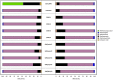
This study investigated the potential of granular activated carbon (GAC) supplementation to enhance anaerobic degradation of dairy wastewater. Two sequential batch reactors (SBRs; 0.8 L working volume), one control and another amended with GAC, were operated at 37°C and 1.5-1.6 m/h upflow velocity for a total of 120 days (four cycles of 30 days each). The methane production at the end of each cycle run increased by about 68%, 503%, 110%, and 125% in the GAC-amended SBR, compared with the Control SBR. Lipid degradation was faster in the presence of GAC. Conversely, the organic compounds, especially lipids, accumulated in the absence of the conductive material. In addition, a reduction in lag phase duration by 46%-100% was observed at all four cycles in the GAC-amended SBR. The peak methane yield rate was at least 2 folds higher with GAC addition in all cycles. RNA-based bacterial analysis revealed enrichment of Synergistes (0.8% to 29.2%) and Geobacter (0.4% to 11.3%) in the GAC-amended SBR. Methanolinea (85.8%) was the dominant archaea in the biofilm grown on GAC, followed by Methanosaeta (11.3%), at RNA level. Overall, this study revealed that GAC supplementation in anaerobic digesters treating dairy wastewater can promote stable and efficient methane production, accelerate lipid degradation and might promote the activity of electroactive microorganisms.
Keywords: conductive materials; dairy wastewater; granular activated carbon; methane production; sequential batch reactor.
© 2022 The Authors. GCB Bioenergy published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Spatial distributions of granular activated carbon in up-flow anaerobic sludge blanket reactors influence methane production treating low and high solid-content wastewater.Bioresour Technol. 2022 Nov;363:127995. doi: 10.1016/j.biortech.2022.127995. Epub 2022 Sep 20. Bioresour Technol. 2022. PMID: 36150426
-
Key syntrophic partnerships identified in a granular activated carbon amended UASB treating municipal sewage under low temperature conditions.Bioresour Technol. 2020 Sep;312:123556. doi: 10.1016/j.biortech.2020.123556. Epub 2020 May 20. Bioresour Technol. 2020. PMID: 32464511
-
RNA-based spatial community analysis revealed intra-reactor variation and expanded collection of direct interspecies electron transfer microorganisms in anaerobic digestion.Bioresour Technol. 2020 Feb;298:122534. doi: 10.1016/j.biortech.2019.122534. Epub 2019 Dec 2. Bioresour Technol. 2020. PMID: 31835200
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
-
Microbial granules on reactors performance during organic butyrate digestion: clean production.Crit Rev Biotechnol. 2023 Dec;43(8):1236-1256. doi: 10.1080/07388551.2022.2103641. Epub 2022 Sep 21. Crit Rev Biotechnol. 2023. PMID: 36130802 Review.
References
-
- APHA . (2017). APHA standard methods for the examination of water and wastewater (23rd ed.), 9780875532875. American Public Health Association.
-
- Baek, G. , Kim, J. , Kim, J. , & Lee, C. (2018). Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies, 11, 107. 10.3390/en11010107 - DOI
LinkOut - more resources
Full Text Sources
