Neoadjuvant Chemotherapy Improves the Immunosuppressive Microenvironment of Bladder Cancer and Increases the Sensitivity to Immune Checkpoint Blockade
- PMID: 35915657
- PMCID: PMC9338739
- DOI: 10.1155/2022/9962397
Neoadjuvant Chemotherapy Improves the Immunosuppressive Microenvironment of Bladder Cancer and Increases the Sensitivity to Immune Checkpoint Blockade
Abstract
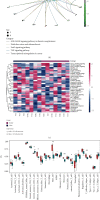
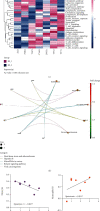
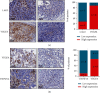
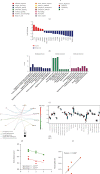
Although tumor immune microenvironment plays an important role in antitumor therapy, few studies explored the gene signatures associated with the tumor immune microenvironment of bladder cancer after neoadjuvant chemotherapy. We examined and analyzed differentially expressed genes from 9 patients with stage I-III bladder cancer by RNA immune-oncology profiling platform. After neoadjuvant chemotherapy, the expressions of 43 genes in 19 pathways and 10 genes in 5 pathways were upregulated and downregulated, respectively. Neoadjuvant chemotherapy also promoted the expression of genes related to the activation of antitumor immune responses and decreased the expression of genes related to tumor proliferation pathways. In addition, neoadjuvant chemotherapy improved tumor response to immune checkpoint blockade. Furthermore, this study also identified several genes that can be used to predict the efficacy of neoadjuvant chemotherapy and their possible molecular mechanisms. In conclusion, neoadjuvant chemotherapy may promote the activation of antitumor effects, improve the suppressive tumor immune microenvironment, and increase the sensitivity of bladder cancer to immune checkpoint blockade.
Copyright © 2022 Hao Luo et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Donat S. M., Shabsigh A., Savage C., et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. European Urology . 2009;55(1):177–186. doi: 10.1016/j.eururo.2008.07.018. - DOI - PubMed
-
- Griffiths G., Hall R., Sylvester R., Raghavan D., Parmar M. K. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. Journal of Clinical Oncology . 2011;29(16):2171–2177. doi: 10.1200/JCO.2010.32.3139. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

