Expression of Long Nonencoding Ribonucleic Acid SNHG20 in Colon Cancer Tissue in Its Influences on Chemotherapeutic Sensitivity of Colon Cancer Cells
- PMID: 35915794
- PMCID: PMC9338858
- DOI: 10.1155/2022/4752782
Expression of Long Nonencoding Ribonucleic Acid SNHG20 in Colon Cancer Tissue in Its Influences on Chemotherapeutic Sensitivity of Colon Cancer Cells
Retraction in
-
Retracted: Expression of Long Nonencoding Ribonucleic Acid SNHG20 in Colon Cancer Tissue in Its Influences on Chemotherapeutic Sensitivity of Colon Cancer Cells.Biomed Res Int. 2024 Jan 9;2024:9798548. doi: 10.1155/2024/9798548. eCollection 2024. Biomed Res Int. 2024. PMID: 38230087 Free PMC article.
Abstract
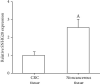
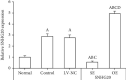
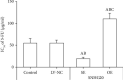
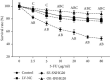
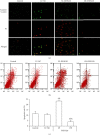
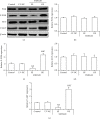
Noncoding RNA (ncRNA) is a kind of RNA that plays a key role in a variety of biological processes, illnesses, and tumours despite the fact that it cannot be translated into proteins. The HT29 colon cancer cell line was utilized to create a 5-FU drug-resistant cell strain (control group), a lentivirus SNHG20 carrier (OE-SNHG20 group), and an SNHG20 shRNA carrier (SNHG20 shRNA carrier group) (SE-SNHG20 group). To determine the expression of cell SNHG20, a real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR) was utilized, and cholecystokinin-octapeptide (CCK-8) was used to detect the difference in 5-FU inhibitory concentration 50. The goal of the study was to see how variations in long nonencoding ribonucleic acid (lncRNA) SNHG20 expression affect colon cancer cell 5-fluorouracil (5-FU) chemotherapeutic sensitivity by collecting colon cancer and normal para cancer tissues and analysing the differences in SNHG20 expression. The ability of cell cladogenesis was tested using platform cladogenesis. Cell apoptosis was detected using flow cytometry. Western blots revealed the presence of protein phosphatidylinositol kinase (PI3K), protein kinase B (AKT), caspase-3, e-cadherin, and matrix metalloproteinase 9 (MMP-9) enzymes. The findings revealed that SNHG20 expression was considerably upregulated (P < 0.05) in colon cancer tissue and 5-FU drug-resistant colon cancer cells. Cell 5-FU IC50, cell cladogenesis, cell survival rate, and MMP-9, P-PI3K, and P-AKT expression were all significantly improved. Cell apoptosis and expressions of E-cadherin and caspase-3, on the other hand, were considerably decreased (P < 0.05). Cell 5-FU IC50, cell cladogenesis, cell survival rate, and the expressions of MMP-9, P-PI3K, and P-AKT were all significantly lower in the SE-SNHG20 group, although cell apoptosis and the expressions of E-cadherin and caspase-3 were significantly higher (P < 0.05). The results revealed that lncRNA SNHG20 could inhibit the chemotherapeutic sensitivity of colon cancer cells to 5-FU by regulating PI3K/AKT pathways. The inhibition of lncRNA SNHG20 expression could promote the apoptosis and proliferation of 5-FU-resistant colon cancer cells.
Copyright © 2022 Wenbin Cao et al.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Influences of LncRNA SNHG20 on proliferation and apoptosis of glioma cells through regulating the PTEN/PI3K/AKT signaling pathway.Eur Rev Med Pharmacol Sci. 2019 Jan;23(1):253-261. doi: 10.26355/eurrev_201901_16771. Eur Rev Med Pharmacol Sci. 2019. PMID: 30657567
-
Downregulation of lncRNA CCAT1 enhances 5-fluorouracil sensitivity in human colon cancer cells.BMC Mol Cell Biol. 2019 Apr 23;20(1):9. doi: 10.1186/s12860-019-0188-1. BMC Mol Cell Biol. 2019. PMID: 31039730 Free PMC article.
-
LncRNA LINC01305 silencing inhibits cell epithelial-mesenchymal transition in cervical cancer by inhibiting TNXB-mediated PI3K/Akt signalling pathway.J Cell Mol Med. 2019 Apr;23(4):2656-2666. doi: 10.1111/jcmm.14161. Epub 2019 Jan 29. J Cell Mol Med. 2019. Retraction in: J Cell Mol Med. 2021 Nov;25(21):10321. doi: 10.1111/jcmm.16942. PMID: 30697971 Free PMC article. Retracted.
-
Long noncoding RNA H19 promotes chemotherapy resistance in choriocarcinoma cells.J Cell Biochem. 2019 Sep;120(9):15131-15144. doi: 10.1002/jcb.28775. Epub 2019 Apr 24. J Cell Biochem. 2019. PMID: 31020694
-
Knockdown of PRDX2 sensitizes colon cancer cells to 5-FU by suppressing the PI3K/AKT signaling pathway.Biosci Rep. 2017 May 11;37(3):BSR20160447. doi: 10.1042/BSR20160447. Print 2017 Jun 30. Biosci Rep. 2017. PMID: 28432271 Free PMC article.
Cited by
-
Retracted: Expression of Long Nonencoding Ribonucleic Acid SNHG20 in Colon Cancer Tissue in Its Influences on Chemotherapeutic Sensitivity of Colon Cancer Cells.Biomed Res Int. 2024 Jan 9;2024:9798548. doi: 10.1155/2024/9798548. eCollection 2024. Biomed Res Int. 2024. PMID: 38230087 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

