WT1 Inhibits Human Renal Carcinoma Cell Proliferation and Induces G2/M Arrest by Upregulating IL-24 Expression
- PMID: 35915803
- PMCID: PMC9338855
- DOI: 10.1155/2022/1093945
WT1 Inhibits Human Renal Carcinoma Cell Proliferation and Induces G2/M Arrest by Upregulating IL-24 Expression
Abstract
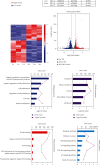
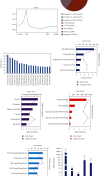
The transcription factor Wilms' tumor 1 (WT1) is involved in development, tissue homeostasis, and disease. However, the exact roles and the mechanisms of WT1 in renal carcinoma are not well understood. Therefore, in this study, we evaluated the ability of WT1 to block proliferation in renal carcinoma cells in vitro. Experimental analysis showed that WT1 overexpression inhibited the proliferation of renal carcinoma A498 cells and promoted arrest at the G2/M checkpoint. RNA-Seq identified differentially expressed genes, including IL-24, related to both the cell proliferation and the cell cycle. WT1 overexpression upregulated IL-24 expression, and IL-24 overexpression induced G2/M arrest. ChIP-Seq identified JUN as a direct target of WT1 in A498 cells, in which positive regulation was shown by RT-qPCR. It has been shown that the transcription factor JUN can regulate IL-24 expression, and therefore, we hypothesize that WT1 might regulate the IL-24 through JUN. Furthermore, analysis based on TCGA datasets showed that the expression of WT1-regulated genes, including TXNIP and GADD45A, was significantly correlated with the stage and histological grade of tumors, with high levels linked to favorable prognoses. Our results demonstrated that the overexpression of WT1 upregulates IL-24, leading to G2/M checkpoint arrest to reduce proliferation. These results indicate that regulation of IL-24 by WT1 inhibits proliferation and may represent a potential target for treating renal carcinoma.
Copyright © 2022 Y. J. Jing et al.
Conflict of interest statement
The authors declare that there are no competing interests.
Figures




Similar articles
-
Wilms' tumor protein induces an epithelial-mesenchymal hybrid differentiation state in clear cell renal cell carcinoma.PLoS One. 2014 Jul 15;9(7):e102041. doi: 10.1371/journal.pone.0102041. eCollection 2014. PLoS One. 2014. PMID: 25025131 Free PMC article.
-
Wilms' tumour 1 can suppress hTERT gene expression and telomerase activity in clear cell renal cell carcinoma via multiple pathways.Br J Cancer. 2010 Oct 12;103(8):1255-62. doi: 10.1038/sj.bjc.6605878. Epub 2010 Sep 14. Br J Cancer. 2010. PMID: 20842112 Free PMC article.
-
Role of the Wilms' tumor 1 gene in the aberrant biological behavior of leukemic cells and the related mechanisms.Oncol Rep. 2014 Dec;32(6):2680-6. doi: 10.3892/or.2014.3529. Epub 2014 Oct 6. Oncol Rep. 2014. PMID: 25310451
-
The role of Wilms' tumor genes.J Med Invest. 1999 Aug;46(3-4):130-40. J Med Invest. 1999. PMID: 10687307 Review.
-
Wilms tumor and the WT1 gene.Exp Cell Res. 2001 Mar 10;264(1):74-99. doi: 10.1006/excr.2000.5131. Exp Cell Res. 2001. PMID: 11237525 Review.
Cited by
-
Immunomodulatory Role of Thioredoxin Interacting Protein in Cancer's Impediments: Current Understanding and Therapeutic Implications.Vaccines (Basel). 2022 Nov 10;10(11):1902. doi: 10.3390/vaccines10111902. Vaccines (Basel). 2022. PMID: 36366411 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

