Nipple Adenoma: Correlation of Imaging Findings and Histopathology
- PMID: 35915844
- PMCID: PMC9334779
- DOI: 10.1093/jbi/wbac019
Nipple Adenoma: Correlation of Imaging Findings and Histopathology
Abstract
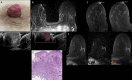
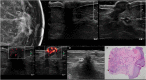
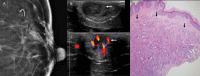
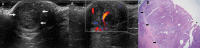
Nipple adenomas (NAs) are benign neoplasms composed of papillary hyperplasia of the epithelium of the major lactiferous ducts. Patients with NA may report bloody nipple discharge and clinically may resemble Paget disease, raising concern for malignancy. Mammographically, NAs are often occult. US can show a hypervascular circumscribed mass centered within the nipple with varying echogenicity. Diagnosis is usually made on punch biopsy or excision, but breast radiologists should be aware of this entity. Malignancy can be found elsewhere in the ipsilateral or contralateral breast, or very rarely may directly extend to involve an NA, but published experience with concurrent malignancies is small. We describe the radiologic-pathologic correlation of NAs.
Keywords: histopathology; nipple adenoma; nipple discharge.
© Society of Breast Imaging 2022. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Nipple adenoma: A clinicopathological study over a period of six years in tertiary care center.Indian J Pathol Microbiol. 2025 Jan 1;68(1):108-112. doi: 10.4103/ijpm.ijpm_742_23. Epub 2024 Aug 8. Indian J Pathol Microbiol. 2025. PMID: 39133250
-
Lesions of the Nipple.Surg Pathol Clin. 2009 Jun;2(2):391-412. doi: 10.1016/j.path.2009.02.010. Epub 2009 Jun 2. Surg Pathol Clin. 2009. PMID: 26838328 Review.
-
Nipple Adenoma: Case Report of a Rare Entity.Cureus. 2022 Mar 9;14(3):e22996. doi: 10.7759/cureus.22996. eCollection 2022 Mar. Cureus. 2022. PMID: 35415057 Free PMC article.
-
Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin.BMC Dermatol. 2016 May 20;16(1):4. doi: 10.1186/s12895-016-0041-6. BMC Dermatol. 2016. PMID: 27206635 Free PMC article.
-
Florid papillomatosis of the nipple: a rare presentation and review of the literature.Breast Dis. 2015;35(2):153-6. doi: 10.3233/BD-150397. Breast Dis. 2015. PMID: 25585841 Review.
Cited by
-
Mammary Paget's Disease Mimicking Benign and Malignant Dermatological Conditions: Clinical Challenges and Diagnostic Considerations.Cureus. 2024 Jul 25;16(7):e65378. doi: 10.7759/cureus.65378. eCollection 2024 Jul. Cureus. 2024. PMID: 39188449 Free PMC article. Review.
-
Syringomatous Tumor of the Nipple.World J Oncol. 2022 Aug;13(4):235-240. doi: 10.14740/wjon1513. Epub 2022 Aug 23. World J Oncol. 2022. PMID: 36128591 Free PMC article.
-
Nipple adenoma detected by multimodal ultrasound: a case report and literature review.Front Oncol. 2024 Oct 23;14:1457293. doi: 10.3389/fonc.2024.1457293. eCollection 2024. Front Oncol. 2024. PMID: 39507760 Free PMC article.
References
-
- Jones DB. Florid papillomatosis of the nipple ducts. Cancer 1955;8(2):315–319. - PubMed
-
- Lester SC, Lee AHS.. Nipple adenoma. In: World Health Organization Classification of Tumours, Breast Tumours. 5th ed. Vol 2. Lyons, France: International Agency for Research on Cancer, 2019:182–183.
-
- Perzin KH, Lattes R.. Papillary adenoma of the nipple (florid papillomatosis, adenoma, adenomatosis). A clinicopathologic study. Cancer 1972;29(4):996–1009. - PubMed
-
- Wang C, Wang X, Ma R.. Diagnosis and surgical treatment of nipple adenoma. ANZ J Surg 2015;85(6):444–447. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
