Extracellular Histones Activate Endothelial NLRP3 Inflammasome and are Associated with a Severe Sepsis Phenotype
- PMID: 35915852
- PMCID: PMC9338392
- DOI: 10.2147/JIR.S363693
Extracellular Histones Activate Endothelial NLRP3 Inflammasome and are Associated with a Severe Sepsis Phenotype
Abstract
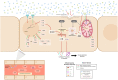
Introduction: Circulating extracellular histones acquire relevance as cytotoxic mediators in sepsis. Extracellular histones act as damage-associated molecular patterns (DAMPs), which induce oxidative stress and NLRP3 inflammasome activation. Inflammasome mediates pyroptosis, a programmed cell death mechanism that produces inflammation. Despite evidence for inflammasome activation in immune cells during sepsis, it was unknown whether extracellular histones can produce endothelial inflammasomes activation.
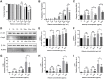
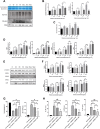
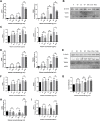
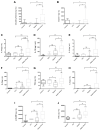
Methods: We used human umbilical vein endothelial cells (HUVEC) to explore the activation of pyroptosis, endothelial function and inflammation by extracellular histones. We evaluated pyroptosis by flow cytometry, caspase-1 activity assay, and gene and protein expression analysis by RT-qPCR and Western blot, respectively. The upstream molecular responses involved in pyroptosis activation by extracellular histones were validated by means of using antioxidant glutathione ethyl ester and NLRP3 inflammasome inhibitors. Finally, using mass spectrometry, we measured circulating histones in blood from critically-ill patients and demonstrated that circulating histone levels correlated with the expression of pyroptosis-related cytokines, the release of endothelial adhesion factors and septic shock severity.
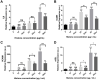
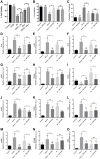
Results: We found that extracellular histones mediate the activation of NLRP3 inflammasome and pyroptosis in endothelial cells by contributing to endothelial dysfunction and the dysregulation of the immune response mediated by endothelium. Likewise, we demonstrated how the hyperacetylation of extracellular histones or the use of antioxidants decreased pyroptosis. In addition, we showed that pyroptosis is a feasible process occurring in septic shock patients.
Discussion: Circulating histone levels correlated with the expression of pro-inflammatory and pyroptosis-related cytokines, the release of endothelial adhesion factors and septic shock severity. We propose to block histone-mediated pyroptosis as a feasible therapeutic strategy in sepsis.
Keywords: NLRP3; endothelium; extracellular histones; histone acetylation; inflammasome; sepsis.
© 2022 Beltrán-García et al.
Conflict of interest statement
Dr José Luis García-Giménez reports grants from Spanish Institute of Health, grants from Ministerio de Ciencia e Innovación, during the conduct of the study; personal fees from EpiDisease S.L., outside the submitted work; In addition, Dr José Luis García-Giménez has a patent EP3535587 licensed to EpiDisease S.L. Dr Carlos Hermenegildo reports grants from University of Valencia, during the conduct of the study. Federico V Pallardó has a pending patent related to this work. The other authors report no conflicts of interest.
Figures
Similar articles
-
Extracellular Histone H3 Induces Pyroptosis During Sepsis and May Act Through NOD2 and VSIG4/NLRP3 Pathways.Front Cell Infect Microbiol. 2020 May 5;10:196. doi: 10.3389/fcimb.2020.00196. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32432055 Free PMC article.
-
Caspase-1 Inflammasome Activation Mediates Homocysteine-Induced Pyrop-Apoptosis in Endothelial Cells.Circ Res. 2016 May 13;118(10):1525-39. doi: 10.1161/CIRCRESAHA.116.308501. Epub 2016 Mar 22. Circ Res. 2016. PMID: 27006445 Free PMC article.
-
BDNF corrects NLRP3 inflammasome-induced pyroptosis and glucose metabolism reprogramming through KLF2/HK1 pathway in vascular endothelial cells.Cell Signal. 2021 Feb;78:109843. doi: 10.1016/j.cellsig.2020.109843. Epub 2020 Nov 27. Cell Signal. 2021. PMID: 33253911
-
Mitochondrial DNA in NLRP3 inflammasome activation.Int Immunopharmacol. 2022 Jul;108:108719. doi: 10.1016/j.intimp.2022.108719. Epub 2022 Mar 26. Int Immunopharmacol. 2022. PMID: 35349960 Review.
-
NLRP3 Inflammasome and Pyroptosis in Liver Pathophysiology: The Emerging Relevance of Nrf2 Inducers.Antioxidants (Basel). 2022 Apr 28;11(5):870. doi: 10.3390/antiox11050870. Antioxidants (Basel). 2022. PMID: 35624734 Free PMC article. Review.
Cited by
-
Emerging therapeutic strategies targeting extracellular histones for critical and inflammatory diseases: an updated narrative review.Front Immunol. 2024 Aug 14;15:1438984. doi: 10.3389/fimmu.2024.1438984. eCollection 2024. Front Immunol. 2024. PMID: 39206200 Free PMC article. Review.
-
Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation.Cells. 2022 Dec 15;11(24):4070. doi: 10.3390/cells11244070. Cells. 2022. PMID: 36552833 Free PMC article.
-
Cell type-specific roles of NLRP3, inflammasome-dependent and -independent, in host defense, sterile necroinflammation, tissue repair, and fibrosis.Front Immunol. 2023 Jul 25;14:1214289. doi: 10.3389/fimmu.2023.1214289. eCollection 2023. Front Immunol. 2023. PMID: 37564649 Free PMC article. Review.
-
Sepsis-Induced Endothelial Dysfunction: Permeability and Regulated Cell Death.J Inflamm Res. 2024 Nov 28;17:9953-9973. doi: 10.2147/JIR.S479926. eCollection 2024. J Inflamm Res. 2024. PMID: 39628705 Free PMC article. Review.
-
The preventative effects of Lactococcus Lactis metabolites against LPS-induced sepsis.Front Microbiol. 2024 Jul 17;15:1404652. doi: 10.3389/fmicb.2024.1404652. eCollection 2024. Front Microbiol. 2024. PMID: 39086654 Free PMC article.
References
-
- Beltrán-García J. Use of circulating histones and their post-translational modifications as biomarkers in sepsis and septic shock [dissertation]; 2022. Available from: https://roderic.uv.es/handle/10550/81440. Accessed July 14, 2022
LinkOut - more resources
Full Text Sources

