A double-blind randomised feasibility trial of angiotensin-2 in cardiac surgery
- PMID: 35915923
- PMCID: PMC9543254
- DOI: 10.1111/anae.15802
A double-blind randomised feasibility trial of angiotensin-2 in cardiac surgery
Abstract
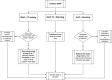
Acute kidney injury is common after cardiac surgery. Vasoplegic hypotension may contribute to kidney injury, and different vasopressors may have variable effects on kidney function. We conducted a double-blind, randomised feasibility trial comparing peri-operative angiotensin-2 with noradrenaline. We randomly allocated 60 patients at two centres to a blinded equipotent angiotensin-2 or noradrenaline infusion intra-operatively and for up to 48 h postoperatively, titrated to mean arterial pressure of 70-80 mmHg. Primary feasibility outcomes included consent rate, protocol adherence, infusion duration, mean arterial pressure maintenance in the target range and major adverse outcomes. Secondary outcomes included kidney injury rate. The consent rate was 47%. Protocol adherence was 100% in the angiotensin-2 group and 94% in the noradrenaline group. Study drug duration was median (IQR [range]) 217 (160-270 [30-315]) vs. 185 (135-301 [0-480]) min (p = 0.78) min intra-operatively, and 5 (0-16 [0-48]) vs. 14.5 (4.8-29 [0-48]) hours (p = 0.075) postoperatively for angiotensin-2 and noradrenaline, respectively. The mean arterial pressure target was achieved postoperatively in 25 of 28 (89%) of the angiotensin-2 group and 27 of 32 (84%) of the noradrenaline group. One participant had a stroke, one required extracorporeal support and three required renal replacement therapy, all in the noradrenaline group (p = 0.99, p = 0.99 and p = 0.1). Acute kidney injury occurred in 7 of 28 in the angiotensin-2 group vs. 12 of 32 patients in the noradrenaline group (p = 0.31). This pilot study suggests that a trial comparing angiotensin-2 with noradrenaline is feasible. Its findings justify further investigations of angiotensin-2 in cardiac surgery.
Keywords: angiotensin-2; cardiac surgery; kidney injury; noradrenaline; norepinephrine; randomised controlled trial; renal dysfunction; renal failure.
© 2022 The Authors. Anaesthesia published by John Wiley & Sons Ltd on behalf of Association of Anaesthetists.
Figures
Comment in
-
Renal failure in cardiac surgery: in search of the magic bullet.Anaesthesia. 2022 Nov;77(11):1197-1201. doi: 10.1111/anae.15857. Epub 2022 Sep 5. Anaesthesia. 2022. PMID: 36059270 No abstract available.
References
-
- Wang Y, Bellomo R. Cardiac surgery‐associated acute kidney injury: risk factors, pathophysiology and treatment. Nature Reviews Nephrology 2017; 13: 697–711. - PubMed
-
- Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long‐term mortality after cardiothoracic surgery. Circulation 2009; 119: 2444–53. - PubMed
-
- Guarracino F, Habicher M, Treskatsch S, et al. Vasopressor therapy in cardiac surgery—an experts' consensus statement. Journal of Cardiothoracic and Vascular Anesthesia 2021; 35: 1018–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical