Developing breast lesion detection algorithms for digital breast tomosynthesis: Leveraging false positive findings
- PMID: 35916103
- PMCID: PMC10156088
- DOI: 10.1002/mp.15883
Developing breast lesion detection algorithms for digital breast tomosynthesis: Leveraging false positive findings
Abstract
Background: Due to the complex nature of digital breast tomosynthesis (DBT) in imaging techniques, reading times are longer than 2D mammograms. A robust computer-aided diagnosis system in DBT could help radiologists reduce their workload and reading times.
Purpose: The purpose of this study was to develop algorithms for detecting biopsy-proven breast lesions on DBT using multi-depth level convolutional models and leveraging non-biopsied samples. As biopsied positive samples in a lesion dataset are limited, we hypothesized that false positive (FP) findings by detection algorithms from non-biopsied benign lesions could improve detection algorithms by using them as data augmentation.
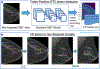
Approach: We first extracted 2D slices from DBT volumes with biopsy-proven breast lesions (cancer and benign), with non-biopsied benign lesions (actionable), and for controls. Then, to provide lesion continuity along the z-direction, we combined a lesion slice with its immediate adjacent slices to synthesize 2.5-dimensional (2.5D) images of the lesion by assigning them into R, G, and B color channels. We used 224 biopsy-proven lesions from 39 cancer and 62 benign patients from a DBTex challenge dataset of 1000 scans. We included the 2.5D images of immediate neighboring slices from the lesion's center to increase the number of training samples. For lesion detection, we used the YOLOv5 algorithm as our base network. We trained a baseline algorithm (medium-depth level) using biopsied samples to detect actionable FPs in non-biopsied images. Afterward, we fine-tuned the baseline model on the augmented image set (actionable FPs added). For lesion inferencing, we processed the DBT volume slice-by-slice to estimate bounding boxes in each slice, and then combined them by connecting bounding boxes along the depth via volumetric morphological closing. We trained an additional model (large) with deeper-depth levels by repeating the above process. Finally, we developed an ensemble algorithm by combining the medium and large detection models. We used the free-response operating characteristic curve to evaluate our algorithms. We reported mean sensitivity per FPs per DBT volume only for biopsied views and sensitivity at 2-false positives per image (2FPI) for all views. However, due to the limited accessibility to the truth of the challenge validation and test datasets, we used sensitivity at 2FPI for statistical evaluation.
Results: For the DBTex independent validation set, the medium baseline model achieved a mean sensitivity of 0.627 FPs per DBT volume, and a sensitivity of 0.640 at 2FPI. After adding actionable FP lesions, the model had an improved 2FPI of 0.769 over the baseline (p-value = 0.013). Our ensemble algorithm with multi-depth levels (medium + large) achieved a mean sensitivity of 0.815 FPs per DBT volume and an improved sensitivity at 2FPI of 0.80 over the baseline (p-value < 0.001) on the validation set. Finally, our ensemble model achieved a mean sensitivity of 0.786 FPs per DBT volume and a sensitivity of 0.743 at 2FPI on the DBTex independent test set.
Conclusions: Our results show that actionable FP findings hold useful information for lesion detection algorithms, and our ensemble detection model with multi-depth levels improves lesion detection performance.
Keywords: breast cancer; computer-aided detection; deep learning; digital breast tomosynthesis; lesion detection.
© 2022 American Association of Physicists in Medicine.
Figures


Similar articles
-
Deep Learning in Digital Breast Tomosynthesis: Current Status, Challenges, and Future Trends.MedComm (2020). 2025 Jun 9;6(6):e70247. doi: 10.1002/mco2.70247. eCollection 2025 Jun. MedComm (2020). 2025. PMID: 40491967 Free PMC article. Review.
-
Multi-domain features for reducing false positives in automated detection of clustered microcalcifications in digital breast tomosynthesis.Med Phys. 2019 Mar;46(3):1300-1308. doi: 10.1002/mp.13394. Epub 2019 Feb 14. Med Phys. 2019. PMID: 30661242
-
Mass detection in digital breast tomosynthesis: Deep convolutional neural network with transfer learning from mammography.Med Phys. 2016 Dec;43(12):6654. doi: 10.1118/1.4967345. Med Phys. 2016. PMID: 27908154 Free PMC article.
-
Detection of masses in digital breast tomosynthesis using complementary information of simulated projection.Med Phys. 2015 Dec;42(12):7043-58. doi: 10.1118/1.4935533. Med Phys. 2015. PMID: 26632059
-
Digital breast tomosynthesis: Image acquisition principles and artifacts.Clin Imaging. 2019 May-Jun;55:188-195. doi: 10.1016/j.clinimag.2018.07.013. Epub 2018 Sep 13. Clin Imaging. 2019. PMID: 30236642 Review.
Cited by
-
The Role of Deep Learning in Advancing Breast Cancer Detection Using Different Imaging Modalities: A Systematic Review.Cancers (Basel). 2022 Oct 29;14(21):5334. doi: 10.3390/cancers14215334. Cancers (Basel). 2022. PMID: 36358753 Free PMC article. Review.
-
The value of predicting breast cancer with a DBT 2.5D deep learning model.Discov Oncol. 2025 Mar 29;16(1):420. doi: 10.1007/s12672-025-02170-6. Discov Oncol. 2025. PMID: 40155449 Free PMC article.
-
An explainable-by-design end-to-end AI framework based on prototypical part learning for lesion detection and classification in Digital Breast Tomosynthesis images.Comput Struct Biotechnol J. 2025 Jun 10;27:2649-2660. doi: 10.1016/j.csbj.2025.06.008. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40599244 Free PMC article.
-
Deep Learning in Digital Breast Tomosynthesis: Current Status, Challenges, and Future Trends.MedComm (2020). 2025 Jun 9;6(6):e70247. doi: 10.1002/mco2.70247. eCollection 2025 Jun. MedComm (2020). 2025. PMID: 40491967 Free PMC article. Review.
-
Cross-Attention Adaptive Feature Pyramid Network with Uncertainty Boundary Modeling for Mass Detection in Digital Breast Tomosynthesis.Bioengineering (Basel). 2025 Feb 17;12(2):196. doi: 10.3390/bioengineering12020196. Bioengineering (Basel). 2025. PMID: 40001715 Free PMC article.
References
-
- Reiser I, Sechopoulos I. A review of digital breast tomosynthesis. Med Phys Int J. 2014;
-
- Deng J, Dong W, Socher R, Li L-J, Kai L, Li F-F. ImageNet: A large-scale hierarchical image database. 2009 IEEE Conference on Computer Vision and Pattern Recognition; 2009;
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

