Outcomes reported in trials of treatments for severe malaria: The need for a core outcome set
- PMID: 35916146
- PMCID: PMC9545330
- DOI: 10.1111/tmi.13803
Outcomes reported in trials of treatments for severe malaria: The need for a core outcome set
Abstract
Objectives: Malaria is one of the most important parasitic infectious diseases worldwide. Despite the scale-up of effective antimalarials, mortality rates from severe malaria (SM) remain significantly high; thus, numerous trials are investigating both antimalarials and adjunctive therapy. This review aimed to summarise all the outcome measures used in trials in the last 10 years to see the need for a core outcome set.
Methods: A systematic review was undertaken to summarise outcomes of individually randomised trials assessing treatments for SM in adults and children. We searched key databases and trial registries between 1 January 2010 and 30 July 2020. Non-randomised trials were excluded to allow comparison of similar trials. Trial characteristics including phase, region, population, interventions, were summarised. All primary and secondary outcomes were extracted and categorised using a taxonomy table.
Results: Twenty-seven of 282 screened trials met our inclusion criteria, including 10,342 patients from 19 countries: 19 (70%) trials from Africa and 8 (30%) from Asia. A large amount of heterogeneity was observed in the selection of outcomes and instruments, with 101 different outcomes measures recorded, 78/101 reported only in a single trial. Parasitological outcomes (17 studies), neurological status (14 studies), death (14 studies) and temperature (10 studies), were the most reported outcomes. Where an outcome was reported in >1 study it was often measured differently: temperature (4 different measures), renal function (7 measures), nervous system (13 measures) and parasitology (10 measures).
Conclusion: Outcomes used in SM trials are inconsistent and heterogeneous. Absence of consensus for outcome measures used impedes research synthesis and comparability of different interventions. This systematic review demonstrates the need to develop a standardised collection of core outcomes for clinical trials of treatments for SM and next steps to include the development of a panel of experts in the field, a Delphi process, and a consensus meeting.
Keywords: core outcome set; outcome measures; severe malaria; systematic review; treatment.
© 2022 The Authors Tropical Medicine & International Health Published by John Wiley & Sons Ltd.
Figures

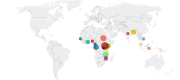
 Uganda (n = 7),
Uganda (n = 7),  Malawi (n = 4),
Malawi (n = 4),  Sudan (n = 3),
Sudan (n = 3),  Congo (n = 3),
Congo (n = 3),  Kenya (n = 3),
Kenya (n = 3),  Mozambique (n = 2),
Mozambique (n = 2),  Ghana (n = 2),
Ghana (n = 2),  Bangladesh (n = 3),
Bangladesh (n = 3),  India (n = 1),
India (n = 1),  Cameroon (n = 1),
Cameroon (n = 1),  Malaysia (n = 1),
Malaysia (n = 1),  Vietnam (n = 1),
Vietnam (n = 1),  Thailand (n = 1),
Thailand (n = 1),  Tanzania (n = 1),
Tanzania (n = 1),  Indonesia (n = 1),
Indonesia (n = 1),  Gambia (n = 1),
Gambia (n = 1),  Nigeria (n = 1),
Nigeria (n = 1),  Rwanda (n = 1),
Rwanda (n = 1),  Gabon (n = 1). The Democratic Republic of the Congo (DRC) and the Republic of Congo are grouped together under Congo
Gabon (n = 1). The Democratic Republic of the Congo (DRC) and the Republic of Congo are grouped together under Congo
References
-
- Ashley E, Pyae Phyo A, Woodrow C. Malaria. Lancet. 2018;391(10130):1608–21. - PubMed
-
- WHO . Severe malaria. Trop Med Int Health. 2014;19(Suppl 1):7–131. - PubMed
-
- World Health Organization . World malaria report 2018. Geneva: WHO; 2018.
-
- Ghebreyesus TA. The malaria eradication challenge. Lancet. 2019;394:990–1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

