Branched Tertiary Amines from Aldehydes and α-Olefins by Combined Multiphase Tandem Reactions
- PMID: 35916208
- PMCID: PMC9804909
- DOI: 10.1002/chem.202202081
Branched Tertiary Amines from Aldehydes and α-Olefins by Combined Multiphase Tandem Reactions
Abstract
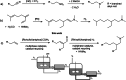
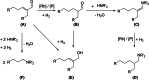
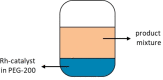
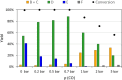
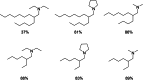
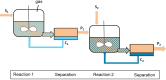
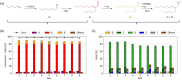
This study presents the transformation of olefins to branched amines by combining a hydroformylation/aldol condensation tandem reaction with the reductive amination in a combined multiphase system that can be recycled 9 times. The products are branched amines that are precursors for surfactants. Since the multiphase hydrofomylation/aldol condensation system has already been studied, the first step was to develop the partial hydrogenation of unsaturated aldehydes together with a subsequent reductive amination. The rhodium/phosphine catalyst is immobilized in a polar polyethylene phase which separates from the product phase after the reaction. Reaction and catalyst recycling are demonstrated by the conversion of the C14 -aldehyde 2-pentylnonenal with the dimethylamine surrogate dimethylammonium dimethylcarbamate to the corresponding tertiary amine with yields up to 88 % and an average rhodium leaching of less than 0.1 % per recycling run. Furthermore, the positive influence of a Bronsted acid and carbon monoxide on the selectivity are discussed. Finally, the two PEG based systems have been merged in one recycling approach, by using the product phase of the hydroformylation aldol condensation reaction for the reductive amination reaction. The yields are stable during a nine recycling runs and the leaching low with 0.09 % over the two recycling stages.
Keywords: amination; homogeneous catalysis; rhodium.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Domino-hydroformylation/aldol condensation catalysis: highly selective synthesis of α,β-unsaturated aldehydes from olefins.Chemistry. 2014 Oct 6;20(41):13210-6. doi: 10.1002/chem.201403302. Epub 2014 Sep 1. Chemistry. 2014. PMID: 25179918
-
Chemo- and regioselective homogeneous rhodium-catalyzed hydroamidomethylation of terminal alkenes to N-alkylamides.ChemSusChem. 2013 Sep;6(9):1759-73. doi: 10.1002/cssc.201300484. Epub 2013 Sep 5. ChemSusChem. 2013. PMID: 24009108
-
Tandem Hydroaminomethylation Reaction to Synthesize Amines from Alkenes.Chem Rev. 2018 Apr 11;118(7):3833-3861. doi: 10.1021/acs.chemrev.7b00667. Epub 2018 Mar 1. Chem Rev. 2018. PMID: 29493233
-
A Multicatalytic Approach to the Hydroaminomethylation of α-Olefins.Angew Chem Int Ed Engl. 2019 Mar 11;58(11):3368-3372. doi: 10.1002/anie.201811297. Epub 2019 Feb 15. Angew Chem Int Ed Engl. 2019. PMID: 30635956 Free PMC article.
-
Teaching Aldehydes New Tricks Using Rhodium- and Cobalt-Hydride Catalysis.Acc Chem Res. 2021 Mar 2;54(5):1236-1250. doi: 10.1021/acs.accounts.0c00771. Epub 2021 Feb 3. Acc Chem Res. 2021. PMID: 33533586 Free PMC article. Review.
References
-
- None
-
- Afanasyev O. I., Kuchuk E., Usanov D. L., Chusov D., Chem. Rev. 2019, 119, 11857–11911; - PubMed
-
- Murugesan K., Senthamarai T., Chandrashekhar V. G., Natte K., Kamer P. C. J., Beller M., Jagadeesh R. V., Chem. Soc. Rev. 2020, 49, 6273–6328; - PubMed
-
- Irrgang T., Kempe R., Chem. Rev. 2020, 120, 9583–9674; - PubMed
-
- Hayes K. S., Appl. Catal. A 2001, 221, 187–195.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

