Extracellular S100B inhibits A-type voltage-gated potassium currents and increases L-type voltage-gated calcium channel activity in dopaminergic neurons
- PMID: 35916350
- PMCID: PMC10738449
- DOI: 10.1002/glia.24254
Extracellular S100B inhibits A-type voltage-gated potassium currents and increases L-type voltage-gated calcium channel activity in dopaminergic neurons
Abstract
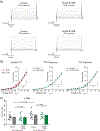
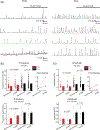
Parkinson's disease (PD) is associated with an increase in secreted S100B within the midbrain and cerebrospinal fluid. In addition, S100B overexpression in mice accelerates the loss of substantia nigra pars compacta dopaminergic (DA) neurons, suggesting a role for this protein in PD pathogenesis. We found that in the mouse SNc, S100B labeled astrocytic processes completely envelop the somata of tyrosine hydroxylase (TH) expressing DA neurons only in male mice. These data suggest that an increase in S100B secretion by astrocytes within the midbrain could play a role in DA dysfunction during early PD. We therefore asked if acute exposure to extracellular S100B alters the activity of identified TH expressing DA neurons in primary mouse midbrain cultures. Acute exposure to 50 pM S100B specifically inhibited A-type voltage-gated potassium currents in TH+ , but not TH- neurons. This was accompanied by ~2-fold increases in the frequency of both intrinsic firing, as well as L-type voltage-gated calcium channel (VGCC)-mediated calcium fluxes only in TH+ neurons. Further, exposure to 100 μM 4-aminopyridine (4-AP), an A-type voltage-gated potassium channel inhibitor, mimicked the S100B mediated increase in intrinsic firing and L-type VGCC-mediated calcium fluxes in TH+ neurons. Taken together, our finding that extracellular S100B alters the activity of native DA neurons via an inhibition of A-type voltage-gated potassium channels has important implications for understanding the pathophysiology of early PD.
Keywords: Parkinson's disease; S100B; astrocytes; calcium channels; neurodegeneration; potassium channels.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
Authors declare no competing financial interests.
Figures






Similar articles
-
6-OHDA induced calcium influx through N-type calcium channel alters membrane properties via PKA pathway in substantia nigra pars compacta dopaminergic neurons.Neurosci Lett. 2014 Jul 11;575:1-6. doi: 10.1016/j.neulet.2014.05.038. Epub 2014 May 23. Neurosci Lett. 2014. PMID: 24861516
-
Compensatory T-type Ca2+ channel activity alters D2-autoreceptor responses of Substantia nigra dopamine neurons from Cav1.3 L-type Ca2+ channel KO mice.Sci Rep. 2015 Sep 18;5:13688. doi: 10.1038/srep13688. Sci Rep. 2015. PMID: 26381090 Free PMC article.
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
-
Lower Affinity of Isradipine for L-Type Ca2+ Channels during Substantia Nigra Dopamine Neuron-Like Activity: Implications for Neuroprotection in Parkinson's Disease.J Neurosci. 2017 Jul 12;37(28):6761-6777. doi: 10.1523/JNEUROSCI.2946-16.2017. Epub 2017 Jun 7. J Neurosci. 2017. PMID: 28592699 Free PMC article.
-
Environment- and activity-dependent dopamine neurotransmitter plasticity in the adult substantia nigra.J Chem Neuroanat. 2016 Apr;73:21-32. doi: 10.1016/j.jchemneu.2015.12.009. Epub 2015 Dec 21. J Chem Neuroanat. 2016. PMID: 26718607 Review.
Cited by
-
How S100B crosses brain barriers and why it is considered a peripheral marker of brain injury.Exp Biol Med (Maywood). 2023 Nov;248(22):2109-2119. doi: 10.1177/15353702231214260. Epub 2023 Dec 6. Exp Biol Med (Maywood). 2023. PMID: 38058025 Free PMC article. Review.
-
Systemically circulating 17β-estradiol enhances the neuroprotective effect of the smoking cessation drug cytisine in female parkinsonian mice.NPJ Parkinsons Dis. 2025 Jan 3;11(1):6. doi: 10.1038/s41531-024-00855-3. NPJ Parkinsons Dis. 2025. PMID: 39753582 Free PMC article.
-
The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker.Int J Mol Sci. 2023 May 31;24(11):9605. doi: 10.3390/ijms24119605. Int J Mol Sci. 2023. PMID: 37298554 Free PMC article. Review.
-
The role of astrocytes from synaptic to non-synaptic plasticity.Front Cell Neurosci. 2024 Oct 18;18:1477985. doi: 10.3389/fncel.2024.1477985. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39493508 Free PMC article. Review.
-
The Role of Astrocytes in Parkinson's Disease : Astrocytes in Parkinson's Disease.Adv Neurobiol. 2024;39:319-343. doi: 10.1007/978-3-031-64839-7_13. Adv Neurobiol. 2024. PMID: 39190081 Review.
References
-
- Baldereschi M, di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, & Inzitari D (2000). Parkinson’s disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. ILSA working group. Italian longitudinal study on aging. Neurology, 55(9), 1358–1363. 10.1212/wnl.55.9.1358 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

