A Variant Allele in Varicella-Zoster Virus Glycoprotein B Selected during Production of the Varicella Vaccine Contributes to Its Attenuation
- PMID: 35916400
- PMCID: PMC9426484
- DOI: 10.1128/mbio.01864-22
A Variant Allele in Varicella-Zoster Virus Glycoprotein B Selected during Production of the Varicella Vaccine Contributes to Its Attenuation
Abstract
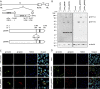
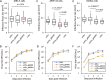
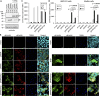
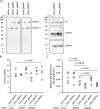
Attenuation of the live varicella Oka vaccine (vOka) has been attributed to mutations in the genome acquired during cell culture passage of pOka (parent strain); however, the precise mechanisms of attenuation remain unknown. Comparative sequence analyses of several vaccine batches showed that over 100 single-nucleotide polymorphisms (SNPs) are conserved across all vaccine batches; 6 SNPs are nearly fixed, suggesting that these SNPs are responsible for attenuation. By contrast, prior analysis of chimeric vOka and pOka recombinants indicates that loci other than these six SNPs contribute to attenuation. Here, we report that pOka consists of a heterogenous population of virus sequences with two nearly equally represented bases, guanine (G) or adenine (A), at nucleotide 2096 of the ORF31 coding sequence, which encodes glycoprotein B (gB) resulting in arginine (R) or glutamine (Q), respectively, at amino acid 699 of gB. By contrast, 2096A/699Q is dominant in vOka (>99.98%). gB699Q/gH/gL showed significantly less fusion activity than gB699R/gH/gL in a cell-based fusion assay. Recombinant pOka with gB669Q (rpOka_gB699Q) had a similar growth phenotype as vOka during lytic infection in cell culture including human primary skin cells; however, rpOka_gB699R showed a growth phenotype similar to pOka. rpOka_gB699R entered neurons from axonal terminals more efficiently than rpOka_gB699Q in the presence of cell membrane-derived vesicles containing gB. Strikingly, when a mixture of pOka with both alleles equally represented was used to infect human neurons from axon terminals, pOka with gB699R was dominant for virus entry. These results identify a variant allele in gB that contributes to attenuation of vOka. IMPORTANCE The live-attenuated varicella vaccine has reduced the burden of chickenpox. Despite its development in 1974, the molecular basis for its attenuation is still not well understood. Since the live-attenuated varicella vaccine is the only licensed human herpesvirus vaccine that prevents primary disease, it is important to understand the mechanism for its attenuation. Here we identify that a variant allele in glycoprotein B (gB) selected during generation of the varicella vaccine contributes to its attenuation. This variant is impaired for fusion, virus entry into neurons from nerve terminals, and replication in human skin cells. Identification of a variant allele in gB, one of the essential herpesvirus core genes, that contributes to its attenuation may provide insights that assist in the development of other herpesvirus vaccines.
Keywords: attenuation mechanism; glycoprotein B; live attenuated varicella vaccine; varicella-zoster virus; virus fusogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The live attenuated varicella-zoster virus vaccine vOka: Molecular and cellular biology of its skin attenuation.Hum Vaccin Immunother. 2025 Dec;21(1):2482286. doi: 10.1080/21645515.2025.2482286. Epub 2025 Mar 28. Hum Vaccin Immunother. 2025. PMID: 40153527 Free PMC article. Review.
-
Varicella Vaccine: a Molecular Variant That May Contribute to Attenuation.mBio. 2022 Dec 20;13(6):e0312022. doi: 10.1128/mbio.03120-22. Epub 2022 Dec 5. mBio. 2022. PMID: 36468883 Free PMC article.
-
Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model.Virology. 2005 Feb 5;332(1):337-46. doi: 10.1016/j.virol.2004.10.047. Virology. 2005. PMID: 15661165
-
Comparison of the Whole-Genome Sequence of an Oka Varicella Vaccine from China with Other Oka Vaccine Strains Reveals Sites Putatively Critical for Vaccine Efficacy.J Virol. 2019 Apr 17;93(9):e02281-18. doi: 10.1128/JVI.02281-18. Print 2019 May 1. J Virol. 2019. PMID: 30728261 Free PMC article.
-
Clinical and molecular aspects of the live attenuated Oka varicella vaccine.Rev Med Virol. 2014 Jul;24(4):254-73. doi: 10.1002/rmv.1789. Epub 2014 Mar 29. Rev Med Virol. 2014. PMID: 24687808 Review.
Cited by
-
Creating the "Dew Drop on a Rose Petal": the Molecular Pathogenesis of Varicella-Zoster Virus Skin Lesions.Microbiol Mol Biol Rev. 2023 Sep 26;87(3):e0011622. doi: 10.1128/mmbr.00116-22. Epub 2023 Jun 24. Microbiol Mol Biol Rev. 2023. PMID: 37354037 Free PMC article. Review.
-
Sequence analysis of isolated strains of herpes zoster virus among patients with shingles.Iran J Microbiol. 2024 Aug;16(4):524-535. doi: 10.18502/ijm.v16i4.16312. Iran J Microbiol. 2024. PMID: 39267939 Free PMC article.
-
Upregulation of keratin 15 is required for varicella-zoster virus replication in keratinocytes and is attenuated in the live attenuated vOka vaccine strain.Virol J. 2024 Oct 9;21(1):253. doi: 10.1186/s12985-024-02514-8. Virol J. 2024. PMID: 39385182 Free PMC article.
-
The live attenuated varicella-zoster virus vaccine vOka: Molecular and cellular biology of its skin attenuation.Hum Vaccin Immunother. 2025 Dec;21(1):2482286. doi: 10.1080/21645515.2025.2482286. Epub 2025 Mar 28. Hum Vaccin Immunother. 2025. PMID: 40153527 Free PMC article. Review.
-
Varicella Vaccine: a Molecular Variant That May Contribute to Attenuation.mBio. 2022 Dec 20;13(6):e0312022. doi: 10.1128/mbio.03120-22. Epub 2022 Dec 5. mBio. 2022. PMID: 36468883 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

