High Levels of Cyclic Diguanylate Interfere with Beneficial Bacterial Colonization
- PMID: 35916402
- PMCID: PMC9426504
- DOI: 10.1128/mbio.01671-22
High Levels of Cyclic Diguanylate Interfere with Beneficial Bacterial Colonization
Abstract
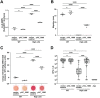
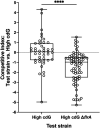
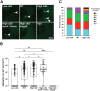
During colonization of the Hawaiian bobtail squid (Euprymna scolopes), Vibrio fischeri bacteria undergo a lifestyle transition from a planktonic motile state in the environment to a biofilm state in host mucus. Cyclic diguanylate (c-di-GMP) is a cytoplasmic signaling molecule that is important for regulating motility-biofilm transitions in many bacterial species. V. fischeri encodes 50 proteins predicted to synthesize and/or degrade c-di-GMP, but a role for c-di-GMP regulation during host colonization has not been investigated. We examined strains exhibiting either low or high levels of c-di-GMP during squid colonization and found that while a low-c-di-GMP strain had no colonization defect, a high c-di-GMP strain was severely impaired. Expression of a heterologous c-di-GMP phosphodiesterase restored colonization, demonstrating that the effect is due to high c-di-GMP levels. In the constitutive high-c-di-GMP state, colonizing V. fischeri exhibited reduced motility, altered biofilm aggregate morphology, and a regulatory interaction where transcription of one polysaccharide locus is inhibited by the presence of the other polysaccharide. Our results highlight the importance of proper c-di-GMP regulation during beneficial animal colonization, illustrate multiple pathways regulated by c-di-GMP in the host, and uncover an interplay of multiple exopolysaccharide systems in host-associated aggregates. IMPORTANCE There is substantial interest in studying cyclic diguanylate (c-di-GMP) in pathogenic and environmental bacteria, which has led to an accepted paradigm in which high c-di-GMP levels promote biofilm formation and reduce motility. However, considerably less focus has been placed on understanding how this compound contributes to beneficial colonization. Using the Vibrio fischeri-Hawaiian bobtail squid study system, we took advantage of recent genetic advances in the bacterium to modulate c-di-GMP levels and measure colonization and track c-di-GMP phenotypes in a symbiotic interaction. Studies in the animal host revealed a c-di-GMP-dependent genetic interaction between two distinct biofilm polysaccharides, Syp and cellulose, that was not evident in culture-based studies: elevated c-di-GMP altered the composition and abundance of the in vivo biofilm by decreasing syp transcription due to increased cellulose synthesis. This study reveals important parallels between pathogenic and beneficial colonization and additionally identifies c-di-GMP-dependent regulation that occurs specifically in the squid host.
Keywords: Euprymna scolopes; Hawaiian bobtail squid; Vibrio fischeri; bacterial colonization; biofilm; c-di-GMP; cyclic diguanylate; flagellar motility; microbiome; motility; squid; symbiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Vibrio fischeri: a model for host-associated biofilm formation.J Bacteriol. 2024 Feb 22;206(2):e0037023. doi: 10.1128/jb.00370-23. Epub 2024 Jan 25. J Bacteriol. 2024. PMID: 38270381 Free PMC article. Review.
-
Functional analysis of cyclic diguanylate-modulating proteins in Vibrio fischeri.mSystems. 2024 Nov 19;9(11):e0095624. doi: 10.1128/msystems.00956-24. Epub 2024 Oct 22. mSystems. 2024. PMID: 39436151 Free PMC article.
-
Para-Aminobenzoic Acid, Calcium, and c-di-GMP Induce Formation of Cohesive, Syp-Polysaccharide-Dependent Biofilms in Vibrio fischeri.mBio. 2021 Oct 26;12(5):e0203421. doi: 10.1128/mBio.02034-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607467 Free PMC article.
-
Calcium-Responsive Diguanylate Cyclase CasA Drives Cellulose-Dependent Biofilm Formation and Inhibits Motility in Vibrio fischeri.mBio. 2021 Dec 21;12(6):e0257321. doi: 10.1128/mBio.02573-21. Epub 2021 Nov 9. mBio. 2021. PMID: 34749532 Free PMC article.
-
Insights into flagellar function and mechanism from the squid-vibrio symbiosis.NPJ Biofilms Microbiomes. 2019 Oct 25;5(1):32. doi: 10.1038/s41522-019-0106-5. eCollection 2019. NPJ Biofilms Microbiomes. 2019. PMID: 31666982 Free PMC article. Review.
Cited by
-
Vibrio fischeri: a model for host-associated biofilm formation.J Bacteriol. 2024 Feb 22;206(2):e0037023. doi: 10.1128/jb.00370-23. Epub 2024 Jan 25. J Bacteriol. 2024. PMID: 38270381 Free PMC article. Review.
-
The enhanced expression of genes encoding diguanylate cyclases under cold stress contributes to the adhesion and biofilm formation of Shewanella putrefaciens WS13.Front Nutr. 2022 Dec 1;9:1076932. doi: 10.3389/fnut.2022.1076932. eCollection 2022. Front Nutr. 2022. PMID: 36532539 Free PMC article.
-
The global regulation of c-di-GMP and cAMP in bacteria.mLife. 2024 Mar 11;3(1):42-56. doi: 10.1002/mlf2.12104. eCollection 2024 Mar. mLife. 2024. PMID: 38827514 Free PMC article. Review.
-
Cyclic Diguanylate in the Wild: Roles During Plant and Animal Colonization.Annu Rev Microbiol. 2024 Nov;78(1):533-551. doi: 10.1146/annurev-micro-041522-101729. Epub 2024 Nov 7. Annu Rev Microbiol. 2024. PMID: 39270684 Free PMC article. Review.
-
Transcriptional pathways across colony biofilm models in the symbiont Vibrio fischeri.mSystems. 2024 Jan 23;9(1):e0081523. doi: 10.1128/msystems.00815-23. Epub 2023 Dec 21. mSystems. 2024. PMID: 38126773 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

