Platelet-Inspired Intravenous Nanomedicine for Injury-Targeted Direct Delivery of Thrombin to Augment Hemostasis in Coagulopathies
- PMID: 35916497
- PMCID: PMC10195184
- DOI: 10.1021/acsnano.2c05306
Platelet-Inspired Intravenous Nanomedicine for Injury-Targeted Direct Delivery of Thrombin to Augment Hemostasis in Coagulopathies
Abstract
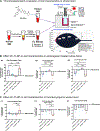
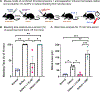
Severe hemorrhage associated with trauma, surgery, and congenital or drug-induced coagulopathies can be life-threatening and requires rapid hemostatic management via topical, intracavitary, or intravenous routes. For injuries that are not easily accessible externally, intravenous hemostatic approaches are needed. The clinical gold standard for this is transfusion of blood products, but due to donor dependence, specialized storage requirements, high risk of contamination, and short shelf life, blood product use faces significant challenges. Consequently, recent research efforts are being focused on designing biosynthetic intravenous hemostats, using intravenous nanoparticles and polymer systems. Here we report on the design and evaluation of thrombin-loaded injury-site-targeted lipid nanoparticles (t-TLNPs) that can specifically localize at an injury site via platelet-mimetic anchorage to the von Willebrand factor (vWF) and collagen and directly release thrombin via diffusion and phospholipase-triggered particle destabilization, which can locally augment fibrin generation from fibrinogen for hemostatic action. We evaluated t-TLNPs in vitro in human blood and plasma, where hemostatic defects were created by platelet depletion and anticoagulation. Spectrophotometric studies of fibrin generation, rotational thromboelastometry (ROTEM)-based studies of clot viscoelasticity, and BioFlux-based real-time imaging of fibrin generation under simulated vascular flow conditions confirmed that t-TLNPs can restore fibrin in hemostatic dysfunction settings. Finally, the in vivo feasibility of t-TLNPs was tested by prophylactic administration in a tail-clip model and emergency administration in a liver-laceration model in mice with induced hemostatic defects. Treatment with t-TLNPs was able to significantly reduce bleeding in both models. Our studies demonstrate an intravenous nanomedicine approach for injury-site-targeted direct delivery of thrombin to augment hemostasis.
Keywords: Fibrin; Hemostasis; Nanoparticle; Platelets; Targeted delivery; Thrombin.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.S.G. is an inventor and co-founder of Haima Therapeutics LLC, a biotechnology start-up company focused on the research and development of bioinspired hemostatic technologies. M.D.N. is a scientific advisory board member of Haima Therapeutics LLC. A.S.G. is a co-inventor on patents US 9107845, US 9636383, US 10426820, US 10434149 that are licensed to Haima.
Figures





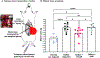
References
-
- Bulger EM; Snyder D; Schoelles K; Gotschall C; Dawson D; Lang E; Sanddal ND; Butler FK; Fallat M; Taillac P; White L; Salomone JP; Seifarth W; Betzner MJ; Johannigman J; McSwain N. An evidence-based prehospital guideline for external hemorrhage control: American College of Surgeons Committee on Trauma. Prehospital Emergency Care 2014, 18, 163–173. - PubMed
-
- Eastridge BJ; Holcomb JB; Shackelford S. Outcomes of traumatic hemorrhagic shock and epidemiology of preventable death from injury. Transfusion 2019, 59, 1423–1428. - PubMed
-
- Buehner MF; Eastridge BJ; Aden JK; DuBose JJ; Blackbourne LH; Cestro RF Combat casualties and severe shock: Risk factors for death at Role 3 military facilities. MILITARY MEDICINE 2017, 182 (9), e1922. - PubMed
-
- Spinella PC; Cap AP Prehospital hemostatic resuscitation to achieve zero preventable deaths after traumatic injury. Curr. Opin. Hematol 2017, 24, 529–535. - PubMed
-
- Klok FA; Kooiman J; Huisman MV; Konstantinides S; Lankeit M. Predicting anticoagulant-related bleeding in patients with venous thromboembolism: A clinically oriented review. Eur. Respir. J 2015, 45, 201–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

