The Proteome of Extracellular Vesicles Produced by the Human Gut Bacteria Bacteroides thetaiotaomicron In Vivo Is Influenced by Environmental and Host-Derived Factors
- PMID: 35916501
- PMCID: PMC9397113
- DOI: 10.1128/aem.00533-22
The Proteome of Extracellular Vesicles Produced by the Human Gut Bacteria Bacteroides thetaiotaomicron In Vivo Is Influenced by Environmental and Host-Derived Factors
Abstract
Bacterial extracellular vesicles (BEVs) released from both Gram-negative and Gram-positive bacteria provide an effective means of communication and trafficking of cell signaling molecules. In the gastrointestinal tract (GIT) BEVs produced by members of the intestinal microbiota can impact host health by mediating microbe-host cell interactions. A major unresolved question, however, is what factors influence the composition of BEV proteins and whether the host influences protein packaging into BEVs and secretion into the GIT. To address this, we have analyzed the proteome of BEVs produced by the major human gut symbiont Bacteroides thetaiotaomicron both in vitro and in vivo in the murine GIT in order to identify proteins specifically enriched in BEVs produced in vivo. We identified 113 proteins enriched in BEVs produced in vivo, the majority (62/113) of which accumulated in BEVs in the absence of any changes in their expression by the parental cells. Among these selectively enriched proteins, we identified dipeptidyl peptidases and an asparaginase and confirmed their increased activity in BEVs produced in vivo. We also showed that intact BEVs are capable of degrading bile acids via a bile salt hydrolase. Collectively these findings provide additional evidence for the dynamic interplay of host-microbe interactions in the GIT and the existence of an active mechanism to drive and enrich a selected group of proteins for secretion into BEVs in the GIT. IMPORTANCE The gastrointestinal tract (GIT) harbors a complex community of microbes termed the microbiota that plays a role in maintaining the host's health and wellbeing. How this comes about and the nature of microbe-host cell interactions in the GIT is still unclear. Recently, nanosized vesicles naturally produced by bacterial constituents of the microbiota have been shown to influence responses of different host cells although the molecular basis and identity of vesicle-born bacterial proteins that mediate these interactions is unclear. We show here that bacterial extracellular vesicles (BEVs) produced by the human symbiont Bacteroides thetaiotaomicron in the GIT are enriched in a set of proteins and enzymes, including dipeptidyl peptidases, an asparaginase and a bile salt hydrolase that can influence host cell biosynthetic pathways. Our results provide new insights into the molecular basis of microbiota-host interactions that are central to maintaining GIT homeostasis and health.
Keywords: Bacteroides thetaiotaomicron; bacterial extracellular vesicles; intestine; microbiota; proteome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
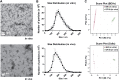
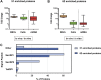
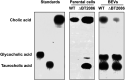

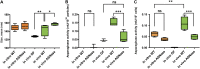
Similar articles
-
Proteomics of Bacterial and Mouse Extracellular Vesicles Released in the Gastrointestinal Tracts of Nutrient-Stressed Animals Reveals an Interplay Between Microbial Serine Proteases and Mammalian Serine Protease Inhibitors.Int J Mol Sci. 2025 Apr 25;26(9):4080. doi: 10.3390/ijms26094080. Int J Mol Sci. 2025. PMID: 40362319 Free PMC article.
-
Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a cell-type specific manner that are altered in inflammatory bowel disease.J Extracell Vesicles. 2022 Jan;11(1):e12189. doi: 10.1002/jev2.12189. J Extracell Vesicles. 2022. PMID: 35064769 Free PMC article.
-
Gut microbiome-derived bacterial extracellular vesicles in patients with solid tumours.J Adv Res. 2025 Feb;68:375-386. doi: 10.1016/j.jare.2024.03.003. Epub 2024 Mar 7. J Adv Res. 2025. PMID: 38458256 Free PMC article.
-
Diet, commensal microbiota-derived extracellular vesicles, and host immunity.Eur J Immunol. 2023 Jul;53(7):e2250163. doi: 10.1002/eji.202250163. Epub 2023 May 17. Eur J Immunol. 2023. PMID: 37137164 Review.
-
Microbiota-Derived Extracellular Vesicle as Emerging Actors in Host Interactions.Int J Mol Sci. 2024 Aug 9;25(16):8722. doi: 10.3390/ijms25168722. Int J Mol Sci. 2024. PMID: 39201409 Free PMC article. Review.
Cited by
-
Iron acquisition by a commensal bacterium modifies host nutritional immunity during Salmonella infection.Cell Host Microbe. 2023 Oct 11;31(10):1639-1654.e10. doi: 10.1016/j.chom.2023.08.018. Epub 2023 Sep 29. Cell Host Microbe. 2023. PMID: 37776864 Free PMC article.
-
Hemophore-like proteins of the HmuY family in the oral and gut microbiome: unraveling the mystery of their evolution.Microbiol Mol Biol Rev. 2024 Mar 27;88(1):e0013123. doi: 10.1128/mmbr.00131-23. Epub 2024 Feb 2. Microbiol Mol Biol Rev. 2024. PMID: 38305743 Free PMC article. Review.
-
A Possible Aquatic Origin of the Thiaminase TenA of the Human Gut Symbiont Bacteroides thetaiotaomicron.J Mol Evol. 2023 Aug;91(4):482-491. doi: 10.1007/s00239-023-10101-8. Epub 2023 Apr 6. J Mol Evol. 2023. PMID: 37022443 Free PMC article.
-
Interactions between extracellular vesicles and microbiome in human diseases: New therapeutic opportunities.Imeta. 2023 Feb 6;2(2):e86. doi: 10.1002/imt2.86. eCollection 2023 May. Imeta. 2023. PMID: 38868436 Free PMC article. Review.
-
Loss of Bacteroides thetaiotaomicron bile acid-altering enzymes impacts bacterial fitness and the global metabolic transcriptome.Microbiol Spectr. 2024 Jan 11;12(1):e0357623. doi: 10.1128/spectrum.03576-23. Epub 2023 Nov 29. Microbiol Spectr. 2024. PMID: 38018975 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- BB/J004529/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/CSP17270/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F000PR10355/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

