Microbiota of the Pregnant Mouse: Characterization of the Bacterial Communities in the Oral Cavity, Lung, Intestine, and Vagina through Culture and DNA Sequencing
- PMID: 35916526
- PMCID: PMC9430855
- DOI: 10.1128/spectrum.01286-22
Microbiota of the Pregnant Mouse: Characterization of the Bacterial Communities in the Oral Cavity, Lung, Intestine, and Vagina through Culture and DNA Sequencing
Abstract
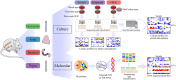
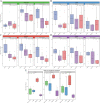
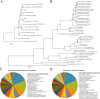
Mice are frequently used as animal models for mechanistic studies of infection and obstetrical disease, yet characterization of the murine microbiota during pregnancy is lacking. The objective of this study was to characterize the microbiotas of distinct body sites of the pregnant mouse-vagina, oral cavity, intestine, and lung-that harbor microorganisms that could potentially invade the murine amniotic cavity, thus leading to adverse pregnancy outcomes. The microbiotas of these body sites were characterized through anoxic, hypoxic, and oxic culture as well as through 16S rRNA gene sequencing. With the exception of the vagina, the cultured microbiotas of each body site varied by atmosphere, with the greatest diversity in the cultured microbiota appearing under anoxic conditions. Only cultures of the vagina were comprehensively representative of the microbiota observed through direct DNA sequencing of body site samples, primarily due to the predominance of two Rodentibacter strains. Identified as Rodentibacter pneumotropicus and Rodentibacter heylii, these isolates exhibited predominance patterns similar to those of Lactobacillus crispatus and Lactobacillus iners in the human vagina. Whole-genome sequencing of these Rodentibacter strains revealed shared genomic features, including the ability to degrade glycogen, an abundant polysaccharide in the vagina. In summary, we report body site-specific microbiotas in the pregnant mouse with potential ecological parallels to those of humans. Importantly, our findings indicate that the vaginal microbiotas of pregnant mice can be readily cultured, suggesting that mock vaginal microbiotas can be tractably generated and maintained for experimental manipulation in future mechanistic studies of host vaginal-microbiome interactions. IMPORTANCE Mice are widely utilized as animal models of obstetrical complications; however, the characterization of the murine microbiota during pregnancy has been neglected. Microorganisms from the vagina, oral cavity, intestine, and lung have been found in the intra-amniotic space, where their presence threatens the progression of gestation. Here, we characterized the microbiotas of pregnant mice and established the appropriateness of culture in capturing the microbiota at each site. The high relative abundance of Rodentibacter observed in the vagina is similar to that of Lactobacillus in humans, suggesting potential ecological parallels. Importantly, we report that the vaginal microbiota of the pregnant mouse can be readily cultured under hypoxic conditions, demonstrating that mock microbial communities can be utilized to test the potential ecological parallels between microbiotas in human and murine pregnancy and to evaluate the relevance of the structure of these microbiotas for adverse pregnancy outcomes, especially intra-amniotic infection and preterm birth.
Keywords: Rodentibacter; anoxic; atmosphere; cultivation; hypoxic; microbiome; mouse model; oxic; pregnancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evidence that intra-amniotic infections are often the result of an ascending invasion - a molecular microbiological study.J Perinat Med. 2019 Nov 26;47(9):915-931. doi: 10.1515/jpm-2019-0297. J Perinat Med. 2019. PMID: 31693497 Free PMC article.
-
Vaginal Microbiome Metagenome Inference Accuracy: Differential Measurement Error according to Community Composition.mSystems. 2023 Apr 27;8(2):e0100322. doi: 10.1128/msystems.01003-22. Epub 2023 Mar 28. mSystems. 2023. PMID: 36975801 Free PMC article.
-
No Consistent Evidence for Microbiota in Murine Placental and Fetal Tissues.mSphere. 2020 Feb 26;5(1):e00933-19. doi: 10.1128/mSphere.00933-19. mSphere. 2020. PMID: 32102944 Free PMC article.
-
Lactobacillus crispatus dominant vaginal microbita in pregnancy.Ceska Gynekol. 2020 Winter;85(1):67-70. Ceska Gynekol. 2020. PMID: 32414287 Review. English.
-
Vaginal microbiome.Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English.
Cited by
-
Diet influences community dynamics following vaginal group B streptococcus colonization.Microbiol Spectr. 2024 Jun 4;12(6):e0362323. doi: 10.1128/spectrum.03623-23. Epub 2024 May 9. Microbiol Spectr. 2024. PMID: 38722155 Free PMC article.
-
The gut microbiota of pregnant and non-pregnant female Hipposideros pomona.BMC Microbiol. 2025 Aug 11;25(1):496. doi: 10.1186/s12866-025-04208-9. BMC Microbiol. 2025. PMID: 40790557 Free PMC article.
-
Locally administered nanosuspension increases delivery of estradiol for the treatment of vaginal atrophy in mice.Drug Deliv Transl Res. 2025 Feb;15(2):609-620. doi: 10.1007/s13346-024-01618-6. Epub 2024 May 15. Drug Deliv Transl Res. 2025. PMID: 38748201
-
Gestational diabetes augments group B Streptococcus infection by disrupting maternal immunity and the vaginal microbiota.Nat Commun. 2024 Feb 3;15(1):1035. doi: 10.1038/s41467-024-45336-6. Nat Commun. 2024. PMID: 38310089 Free PMC article.
-
The gut microbiota facilitate their host tolerance to extreme temperatures.BMC Microbiol. 2024 Apr 20;24(1):131. doi: 10.1186/s12866-024-03277-6. BMC Microbiol. 2024. PMID: 38643098 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

