An in vitro and ex vivo wound infection model to test topical and systemic treatment with antibiotics
- PMID: 35916629
- PMCID: PMC9804477
- DOI: 10.1111/jam.15756
An in vitro and ex vivo wound infection model to test topical and systemic treatment with antibiotics
Abstract
Aims: This study aimed to develop a wound infection model that could be used to test antibiotic-loaded electrospun matrices for the topical treatment of infected skin and compare the effectiveness of this treatment to systemically applied antibiotics.
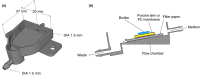
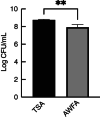
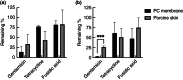
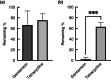
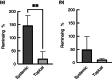
Methods and results: 3D-printed flow chambers were made in which Staphylococcus aureus biofilms were grown either on a polycarbonate membrane or explanted porcine skin. The biofilms were then treated either topically, by placing antibiotic-loaded electrospun matrices on top of the biofilms, or systemically by the addition of antibiotics in the growth medium that flowed underneath the membrane or skin. The medium that was used was either a rich medium or an artificial wound fluid. The results showed that microbial viability in the biofilms was reduced to a greater extent with the topical electrospun matrices when compared to systemic treatment.
Conclusions: An ex vivo infection model was developed that is flexible and can be used to test both topical and systemic treatment of wound infections. It represents a significant improvement over previous in vitro models that we have used to test electrospun membranes.
Significance and impact of the study: The availability of a relatively simple wound infection model in which different delivery methods and dosage regimes can be tested is beneficial for the development of improved treatments for wound infections.
Keywords: Staphylococcus aureus; antibiotics; electrospinning; wound dressing; wound infection model.
© 2022 The Authors. Journal of Applied Microbiology published by John Wiley & Sons Ltd on behalf of Society for Applied Microbiology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Agostinho, A.M. , Hartman, A. , Lipp, C. , Parker, A.E. , Stewart, P.S. & James, G.A. (2011) An in vitro model for the growth and analysis of chronic wound MRSA biofilms. Journal of Applied Microbiology, 111, 1275–1282. - PubMed
-
- Agwuh, K.N. & MacGowan, A. (2006) Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. The Journal of Antimicrobial Chemotherapy, 58, 256–265. - PubMed
-
- Akiyama, H. , Kanzaki, H. , Tada, J. & Arata, J. (1996) Staphylococcus aureus infection on cut wounds in the mouse skin: experimental staphylococcal botryomycosis. Journal of Dermatological Science, 11, 234–238. - PubMed
-
- Alhusein, N. , Blagbrough, I.S. , Beeton, M.L. , Bolhuis, A. & De Bank, P.A. (2016) Electrospun Zein/PCL fibrous matrices release tetracycline in a controlled manner, killing Staphylococcus aureus both in biofilms and ex vivo on pig skin, and are compatible with human skin cells. Pharmaceutical Research, 33, 237–246. - PMC - PubMed
-
- Alhusein, N. , Blagbrough, I.S. & De Bank, P.A. (2013) Zein/polycaprolactone electrospun matrices for localised controlled delivery of tetracycline. Drug Delivery and Translational Research, 3, 542–550. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

