The pathogenesis of cryptoglandular anal fistula: New insight into the immunological profile
- PMID: 35916639
- PMCID: PMC10087133
- DOI: 10.1111/codi.16290
The pathogenesis of cryptoglandular anal fistula: New insight into the immunological profile
Abstract
Aim: The aetiology of cryptoglandular anal fistula (AF) is poorly understood. Evidence suggests that persistence and/or recurrence of the disease is more related to inflammatory than infectious factors. The aim of this study was to investigate the immune profile of cryptoglandular AF and to perform a histopathological characterization.
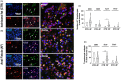
Method: Fistulectomy was performed in all patients; healthy ischioanal fat from the same patients was used as a control. Samples were evaluated by the Luminex xMAP system for the detection of 27 analytes. AF tissues were analysed using immunofluorescence. Staining was performed using primary antibodies to identify M1 inflammatory and M2 anti-inflammatory macrophages. Selective staining of total T lymphocytes and different T lymphocyte subsets was performed.
Results: Twenty patients with AF underwent a fistulectomy. Specific cytokine pathways differentiated AF from healthy tissue: pro-inflammatory cytokines interleukin (IL)-1β, IL-4, IL-8 and IL-17 and the anti-inflammatory cytokine IL-10 were overexpressed in AF compared with controls. Chemokines involved in macrophage recruitment (CCL2, CCL3, CCL4) were higher in AF than in healthy fatty tissue. Moreover, we showed that Tc17 cells characterize AF patients, thus confirming the enzyme-linked immunosorbent assay data. Furthermore, elevated infiltration of CD68+ myeloid cells and a reduction of the M1/M2 ratio characterize AF patients.
Conclusion: A combination of inflammatory cytokines, chemokines and growth factors reside in the wound microenvironment of AF patients. For the first time an important prevalence of Tc17 cells and a reduction in the M1/M2 ratio was observed, thus suggesting new insights into the immunological characterization of AF patients.
Keywords: aetiology; anal fistula; inflammation.
© 2022 The Authors. Colorectal Disease published by John Wiley & Sons Ltd on behalf of Association of Coloproctology of Great Britain and Ireland.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Göttgens KW, Smeets RR, Stassen LP, Beets G, Breukink SO. Systematic review and meta‐analysis of surgical interventions for high cryptoglandular perianal fistula. Int J Colorectal Dis. 2015;30:583–93. - PubMed
-
- Parés D. Pathogenesis and treatment of fistula in ano. Br J Surg. 2011;98:2–3. - PubMed
-
- van Koperen PJ, ten Kate FJ, Bemelman WA, Slors JF. Histological identification of epithelium in perianal fistulae: a prospective study. Colorectal Dis. 2010;12:891–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

