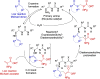
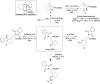
Asymmetric Alkylation of Cyclic Ketones with Dehydroalanine via H-Bond-Directing Enamine Catalysis: Straightforward Access to Enantiopure Unnatural α-Amino Acids
- PMID: 35916657
- PMCID: PMC9805190
- DOI: 10.1002/chem.202201994
Asymmetric Alkylation of Cyclic Ketones with Dehydroalanine via H-Bond-Directing Enamine Catalysis: Straightforward Access to Enantiopure Unnatural α-Amino Acids
Abstract
The growing importance of structurally diverse and functionalized enantiomerically pure unnatural amino acids in the design of drugs, including peptides, has stimulated the development of new synthetic methods. This study reports the challenging direct asymmetric alkylation of cyclic ketones with dehydroalanine derivatives via a conjugate addition reaction for the synthesis of enantiopure ketone-based α-unnatural amino acids. The key to success was the design of a bifunctional primary amine-thiourea catalyst that combines H-bond-directing activation and enamine catalysis. The simultaneous dual activation of the two relatively unreactive partners, confirmed by mass spectrometry studies, results in high reactivity while securing high levels of stereocontrol. A broad substrate scope is accompanied by versatile downstream chemical modifications. The mild reaction conditions and consistently excellent enantioselectivities (>95 % ee in most cases) render this protocol highly practical for the rapid construction of valuable noncanonical enantiopure α-amino-acid building blocks.
Keywords: conjugate addition; ketones; noncovalent Interactions; organocatalysis; unnatural α-amino acids.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Highly diastereoselective and enantioselective Michael addition of 5H-oxazol-4-ones to α,β-unsaturated ketones catalyzed by a new bifunctional organocatalyst with broad substrate scope and applicability.Chem Commun (Camb). 2012 Jan 11;48(3):461-3. doi: 10.1039/c1cc15928c. Epub 2011 Nov 11. Chem Commun (Camb). 2012. PMID: 22075727
-
Highly enantioselective direct conjugate addition of ketones to nitroalkenes promoted by a chiral primary amine-thiourea catalyst.J Am Chem Soc. 2006 Jun 7;128(22):7170-1. doi: 10.1021/ja0620890. J Am Chem Soc. 2006. PMID: 16734464
-
Remote enantioselective Friedel-Crafts alkylations of furans through HOMO activation.Angew Chem Int Ed Engl. 2014 May 19;53(21):5449-52. doi: 10.1002/anie.201403082. Epub 2014 Apr 22. Angew Chem Int Ed Engl. 2014. PMID: 24756964
-
Enamine/Transition Metal Combined Catalysis: Catalytic Transformations Involving Organometallic Electrophilic Intermediates.Top Curr Chem (Cham). 2019 Nov 16;377(6):38. doi: 10.1007/s41061-019-0267-y. Top Curr Chem (Cham). 2019. PMID: 31732819 Free PMC article. Review.
-
[Asymmetric Synthesis of Unnatural Amino Acid-containing Peptides via Direct Asymmetric Reaction of Peptidyl Compounds].Yakugaku Zasshi. 2018;138(11):1371-1379. doi: 10.1248/yakushi.18-00143. Yakugaku Zasshi. 2018. PMID: 30381645 Review. Japanese.
Cited by
-
Enantioselective organocatalytic strategies to access noncanonical α-amino acids.Chem Sci. 2024 Mar 25;15(16):5832-5868. doi: 10.1039/d4sc01081g. eCollection 2024 Apr 24. Chem Sci. 2024. PMID: 38665517 Free PMC article. Review.
-
Giese-type alkylation of dehydroalanine derivatives via silane-mediated alkyl bromide activation.Beilstein J Org Chem. 2024 Dec 17;20:3274-3280. doi: 10.3762/bjoc.20.271. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39717264 Free PMC article.
References
-
- None
-
- Pollegioni L., Servi S., in Unnatural Amino Acids, Springer, New York, 2012;
-
- Hughes A. B., in Amino Acids, Peptides and Proteins in Organic Chemistry, Wiley-VCH, Weinheim, 2009;
-
- Lang K., Chin J. W., Chem. Rev. 2014, 114, 4764–4806; - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources