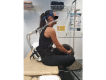
The Utility and Acceptability of a New Noninvasive Ventilatory Assist Device, Rest-Activity Cycler-Positive Airways Pressure, During Exercise in a Population of Healthy Adults: Cohort Study
- PMID: 35916705
- PMCID: PMC9379794
- DOI: 10.2196/35494
The Utility and Acceptability of a New Noninvasive Ventilatory Assist Device, Rest-Activity Cycler-Positive Airways Pressure, During Exercise in a Population of Healthy Adults: Cohort Study
Abstract
Background: Noninvasive ventilation has been demonstrated to benefit people who have moderate to severe chronic obstructive pulmonary disease during acute exacerbations. Studies have begun to investigate the effectiveness of noninvasive ventilation during pulmonary rehabilitation to improve outcomes for people with chronic obstructive pulmonary disease; however, the lack of portability and humidification of these devices means their use is limited, especially when performing activities of daily living. A new prototype device, RACer-PAP (rest-activity cycler-positive airways pressure), delivers battery-operated positive airway pressure via a nasal interface while regulating nasal airway apportionment bias, removing the need for supplementary humidification. This device may offer people with chronic obstructive pulmonary disease an improved ability to participate in pulmonary rehabilitation and activities of daily living.
Objective: To assess the feasibility of exercising with the RACer-PAP in situ and the acceptability of the device during exercise in normal, healthy individuals.
Methods: A total of 15 healthy adults were invited to attend 2 exercise sessions, each 1 week apart. Sessions lasted approximately 1 hour and included 2 baseline 6-minute walk distance assessments, once with and once without the RACer-PAP in situ. Vital signs and spirometry results were monitored throughout, and spirometry was performed pre- and posttesting with RACer-PAP. Subjective questionnaires ascertained participant feedback on exercising with the device in situ.
Results: Of the 15 initial participants, 14 (93%) completed both sessions. There were no adverse events associated with exercising with the device in situ. There were no differences in vital signs or 6-minute walk distance whether exercising with or without the device in situ. There were small increases in maximum dyspnea score (on the Borg scale) when exercising with the device in situ (median score 2.0, IQR 0.5-3.0, vs 3.0, IQR 2.0-3.25). There were small increases in forced vital capacity following exercise with the RACer-PAP. None of the participants reported symptoms associated with airway drying. Participant feedback provided recommendations for modifications for the next iteration of the device prior to piloting the device with people with chronic obstructive pulmonary disease.
Conclusions: This study has shown RACer-PAP to be safe and feasible to use during exercise and has provided feedback for modifications to the device to improve its use during exercise. We now propose to consider the application of the device in a small pilot feasibility study to assess the safety, feasibility, and utility of the device in a population of people with moderate to severe chronic obstructive pulmonary disease.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12619000478112; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375477.
Keywords: COPD; exercise; feasibility; humans; noninvasive ventilation; physiotherapy; pulmonary rehabilitation; rehabilitation.
©Julie Reeve, Sarah Mooney, Nicola Jepsen, David White. Originally published in JMIR Rehabilitation and Assistive Technology (https://rehab.jmir.org), 01.08.2022.
Conflict of interest statement
Conflicts of Interest: DW is listed as co-inventor in the RACer-PAP patent.
Figures
References
-
- Global Strategy For The Diagnosis, Management, And Prevention Of Chronic Obstructive Pulmonary Disease: 2021 Report. Global Initiative for Chronic Obstructive Lung Disease. [2022-06-22]. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25... .
-
- Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H, Chan KY, Sheikh A, Rudan I, Global Health Epidemiology Reference Group (GHERG) Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015 Dec;5(2):020415. doi: 10.7189/jogh.05-020415. http://europepmc.org/abstract/MED/26755942 jogh-05-020415 - DOI - PMC - PubMed
-
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald VM, Phillips K, Smith BJ, Zwar NA, Dabscheck E. COPD-X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med J Aust. 2017 Nov 20;207(10):436–442. doi: 10.5694/mja17.00686.10.5694/mja17.00686 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials