Selective disruption of Drp1-independent mitophagy and mitolysosome trafficking by an Alzheimer's disease relevant tau modification in a novel Caenorhabditis elegans model
- PMID: 35916724
- PMCID: PMC9434186
- DOI: 10.1093/genetics/iyac104
Selective disruption of Drp1-independent mitophagy and mitolysosome trafficking by an Alzheimer's disease relevant tau modification in a novel Caenorhabditis elegans model
Abstract
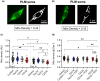
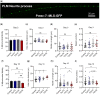
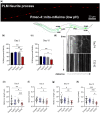
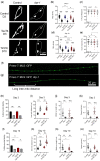
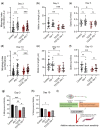
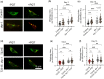
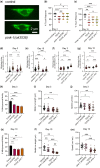
Accumulation of inappropriately phosphorylated tau into neurofibrillary tangles is a defining feature of Alzheimer's disease, with Tau pT231 being an early harbinger of tau pathology. Previously, we demonstrated that expressing a single genomic copy of human phosphomimetic mutant tau (T231E) in Caenorhabditis elegans drove age-dependent neurodegeneration. A critical finding was that T231E, unlike wild-type tau, completely and selectively suppressed oxidative stress-induced mitophagy. Here, we used dynamic imaging approaches to analyze T231E-associated changes in mitochondria and mitolysosome morphology, abundance, trafficking, and stress-induced mitophagy as a function of mitochondrial fission mediator dynamin-related protein 1, which has been demonstrated to interact with hyper phosphorylated tau and contribute to Alzheimer's disease pathogenesis, as well as Pink1, a well-recognized mediator of mitochondrial quality control that works together with Parkin to support stress-induced mitophagy. T231E impacted both mitophagy and mitolysosome neurite trafficking with exquisite selectivity, sparing macroautophagy as well as lysosome and autolysosome trafficking. Both oxidative-stress-induced mitophagy and the ability of T231E to suppress it were independent of drp-1, but at least partially dependent on pink-1. Organelle trafficking was more complicated, with drp-1 and pink-1 mutants exerting independent effects, but generally supported the idea that the mitophagy phenotype is of greater physiologic impact in T231E. Collectively, our results refine the mechanistic pathway through which T231E causes neurodegeneration, demonstrating pathologic selectivity for mutations that mimic tauopathy-associated post-translational modifications, physiologic selectivity for organelles that contain damaged mitochondria, and molecular selectivity for dynamin-related protein 1-independent, Pink1-dependent, perhaps adaptive, and mitophagy.
Keywords: Caenorhabditis elegans; Alzheimer’s disease; Drp1; Pink1; mitochondria; mitophagy; tau phosphorylation.
© The Author(s) 2022. Published by Oxford University Press on behalf of Genetics Society of America. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Tauopathy-associated tau modifications selectively impact neurodegeneration and mitophagy in a novel C. elegans single-copy transgenic model.Mol Neurodegener. 2020 Nov 9;15(1):65. doi: 10.1186/s13024-020-00410-7. Mol Neurodegener. 2020. PMID: 33168053 Free PMC article.
-
Mitochondrial dysfunction, mitophagy, and role of dynamin-related protein 1 in Alzheimer's disease.J Neurosci Res. 2021 Apr;99(4):1120-1135. doi: 10.1002/jnr.24781. Epub 2021 Jan 19. J Neurosci Res. 2021. PMID: 33465841 Review.
-
Deficiencies in mitochondrial dynamics sensitize Caenorhabditis elegans to arsenite and other mitochondrial toxicants by reducing mitochondrial adaptability.Toxicology. 2017 Jul 15;387:81-94. doi: 10.1016/j.tox.2017.05.018. Epub 2017 Jun 8. Toxicology. 2017. PMID: 28602540 Free PMC article.
-
Autophagy facilitates mitochondrial rebuilding after acute heat stress via a DRP-1-dependent process.J Cell Biol. 2021 Apr 5;220(4):e201909139. doi: 10.1083/jcb.201909139. J Cell Biol. 2021. PMID: 33734301 Free PMC article.
-
Are mitophagy enhancers therapeutic targets for Alzheimer's disease?Biomed Pharmacother. 2022 May;149:112918. doi: 10.1016/j.biopha.2022.112918. Epub 2022 Apr 4. Biomed Pharmacother. 2022. PMID: 35585708 Free PMC article. Review.
Cited by
-
The multiple links between actin and mitochondria.Nat Rev Mol Cell Biol. 2023 Sep;24(9):651-667. doi: 10.1038/s41580-023-00613-y. Epub 2023 Jun 5. Nat Rev Mol Cell Biol. 2023. PMID: 37277471 Free PMC article. Review.
-
Tau phosphorylation suppresses oxidative stress-induced mitophagy via FKBP8 receptor modulation.bioRxiv [Preprint]. 2024 Jul 9:2024.07.05.602170. doi: 10.1101/2024.07.05.602170. bioRxiv. 2024. Update in: PLoS One. 2025 Jan 3;20(1):e0307358. doi: 10.1371/journal.pone.0307358. PMID: 39026868 Free PMC article. Updated. Preprint.
-
Tau phosphorylation suppresses oxidative stress-induced mitophagy via FKBP8 receptor modulation.PLoS One. 2025 Jan 3;20(1):e0307358. doi: 10.1371/journal.pone.0307358. eCollection 2025. PLoS One. 2025. PMID: 39752365 Free PMC article.
-
Studying the mechanisms of neurodegeneration: C. elegans advantages and opportunities.Front Cell Neurosci. 2025 Mar 26;19:1559151. doi: 10.3389/fncel.2025.1559151. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40207239 Free PMC article. Review.
-
Drp1: Focus on Diseases Triggered by the Mitochondrial Pathway.Cell Biochem Biophys. 2024 Jun;82(2):435-455. doi: 10.1007/s12013-024-01245-5. Epub 2024 Mar 4. Cell Biochem Biophys. 2024. PMID: 38438751 Review.
References
-
- Aragão Gomes L, Uytterhoeven V, Lopez-Sanmartin D, Tomé SO, Tousseyn T, Vandenberghe R, Vandenbulcke M, von Arnim CAF, Verstreken P, Thal DR, et al.Maturation of neuronal AD-tau pathology involves site-specific phosphorylation of cytoplasmic and synaptic tau preceding conformational change and fibril formation. Acta Neuropathol. 2021;141(2):173–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

