Alcohol acyl transferase genes at a high-flavor intensity locus contribute to ester biosynthesis in kiwifruit
- PMID: 35916752
- PMCID: PMC9516725
- DOI: 10.1093/plphys/kiac316
Alcohol acyl transferase genes at a high-flavor intensity locus contribute to ester biosynthesis in kiwifruit
Abstract
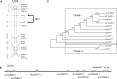
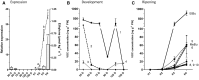
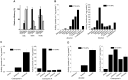
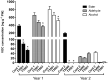
Volatile esters are key compounds contributing to flavor intensity in commonly consumed fruits including apple (Malus domestica), strawberry (Fragaria spp.), and banana (Musa sapientum). In kiwifruit (Actinidia spp.), ethyl butanoate and other esters have been proposed to contribute fruity, sweet notes to commercial cultivars. Here, we investigated the genetic basis for ester production in Actinidia in an A. chinensis mapping population (AcMPO). A major quantitative trait loci for the production of multiple esters was identified at the high-flavor intensity (HiFI) locus on chromosome 20. This locus co-located with eight tandemly arrayed alcohol acyl transferase genes in the Red5 genome that were expressed in a ripening-specific fashion that corresponded with ester production. Biochemical characterization suggested two genes at the HiFI locus, alcohol acyl transferase 16-b/c (AT16-MPb/c), probably contributed most to the production of ethyl butanoate. A third gene, AT16-MPa, probably contributed more to hexyl butanoate and butyl hexanoate production, two esters that segregated in AcMPO. Sensory analysis of AcMPO indicated that fruit from segregating lines with high ester concentrations were more commonly described as being "fruity" as opposed to "beany". The downregulation of AT16-MPa-c by RNAi reduced ester production in ripe "Hort16A" fruit by >90%. Gas chromatography-olfactometry indicated the loss of the major "fruity" notes contributed by ethyl butanoate. A comparison of unimproved Actinidia germplasm with those of commercial cultivars indicated that the selection of fruit with high concentrations of alkyl esters (but not green note aldehydes) was probably an important selection trait in kiwifruit cultivation. Understanding ester production at the HiFI locus is a critical step toward maintaining and improving flavor intensity in kiwifruit.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Atkinson RG, Gunaseelan K, Wang MY, Luo L, Wang T, Norling CL, Johnston SL, Maddumage R, Schröder R, Schaffer RJ (2011) Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using an ACC-oxidase knockdown line. J Exp Bot 62: 3821–3835 - PubMed
-
- Auvray M, Spence C (2008) The multisensory perception of flavor. Conscious Cogn 17: 1016–1031 - PubMed
-
- Baldwin EA, Scott JW, Shewmaker CK, Schuch W (2000) Flavor trivia and tomato aroma: biochemistry and possible mechanisms for control of important aroma components. HortScience 35: 1013–1022
-
- Bauchot A, Mottram D, Dodson A, John P (1998) Effect of aminocyclopropane‐1‐carboxylic acid oxidase antisense gene on the formation of volatile esters in cantaloupe charentais melon (cv. Vedrandais). J Agric Food Chem 46: 4787–4792
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

