The potential effect of leukocyte filtration methods on erythrocyte-derived microvesicles: One step forward
- PMID: 35916762
- PMCID: PMC9580532
- DOI: 10.4081/ejtm.2022.10708
The potential effect of leukocyte filtration methods on erythrocyte-derived microvesicles: One step forward
Abstract
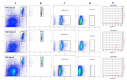
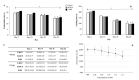
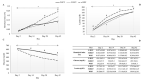
By harmonizing the pre-preparation conditions and also removing some donors' variations, the current study took one step forward to investigate whether different leukocyte filtration sets influence the quality of RBCs throughout the storage time. Twelve whole blood units were collected, and each unit was split into three equal parts. Thirty-six divided bags were filtered using three different leukocyte-filtration sets including Red Cell and Whole Blood Filters (12 units per filter). The prepared RBCs were refrigerated for up to 42 days and assessed for microvesicle count and size, clotting- and prothrombin time, hemolysis index, and biochemical parameters. A significant increment in erythrocytes microvesicle count (EMVs/μL) was observed during the time in the three filtration sets. The number of EMVs in WBF-RBCs was higher (~1.6 fold) than in F-RCF on day 42 (p=0.035). Interestingly the median fluorescence intensity of EMVs decreased during the storage. The size of MVs rose during the time without any significant differences among the filters. Coagulation time decreased in RBCs over the storage, with no significant differences among the filters. Hemolysis index and lactate concentration increased while glucose level decreased significantly throughout the time. The changes in WBF-RBCs were more drastic rather than RCF-RBCs. The only significant difference in the count of EMVs was between WBF and F-RCF components on day 42. Though the changes in WBF products were more drastic, all the values fell within the standard limits. Accordingly, all three filtration sets can be considered.
Conflict of interest statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
During storage, erythrocytes undergo a variety of detrimental oxidative, biochemical, and biomechanical alterations collectively denoted as “hypothermal storage lesions”. Exposure to various stressors makes erythrocytes susceptible to destruction, hemolysis, and several membrane alterations, which end up with the formation of microvesicles (MVs).,MVs are submicron membrane-covered particles heterogeneous in size (50 nm-1 μm) and originated from different cells under various circumstances in health and disease. They carry their parents' molecular contents and cell-specific markers by which they are characterized. As a permanent feature of the aging process, vesiculation occurs in both in and ex vivo conditions. Actually, in the context of blood transfusion erythrocytes-derived microvesicles (EMVs) are assumed as storage lesions.
Growing documents have signified they are involved in a broad spectrum of physiological and pathological mechanisms and may boost some pathways, including coagulation and inflammation.,,, In addition to storage conditions, variability of donors, pre-processing conditions, and blood processing techniques are referred to as sources of RBCs quality variations associated with undesired clinical consequences and poor outcomes.,,, As regards, the issued RBCs produced through different preparation procedures are not equally processed, even though the whole process is tightly supervised, elucidating the role of blood preparation methods in the quality of the components is crucially important., At the Iranian Blood Transfusion Organization (IBTO), the leukocyte-filtration process began in 2005, and today about one-third of the RBCs components are produced using two different leukocyte filtration procedures including whole blood- (WBF) and red cell (RCF) filtration. Regarding the emphasis on using leukocyte-filtered products, the current experimental study was established to investigate the possible effects of three different leukocyte-depletion filters (which are currently used at IBTO) on the EMVs formation in RBCs components and also the quality of the components by harmonizing the preparation conditions and eliminating some confounding variables.
Figures

Similar articles
-
Quantification of Cell-Free DNA in Red Blood Cell Units in Different Whole Blood Processing Methods.J Blood Transfus. 2016;2016:9316385. doi: 10.1155/2016/9316385. Epub 2016 Sep 27. J Blood Transfus. 2016. PMID: 27774338 Free PMC article.
-
Impact of various red cell concentrate preparation methods on the efficiency of prestorage white cell filtration and on red cells during storage for 42 days.Transfusion. 1997 Nov-Dec;37(11-12):1137-42. doi: 10.1046/j.1537-2995.1997.37111298088042.x. Transfusion. 1997. PMID: 9426636
-
Characteristics of Extracellular Vesicles in Red Blood Concentrates Change with Storage Time and Blood Manufacturing Method.Transfus Med Hemother. 2018 May;45(3):185-193. doi: 10.1159/000486137. Epub 2018 Mar 16. Transfus Med Hemother. 2018. PMID: 29928174 Free PMC article.
-
Prestorage WBC filtration of RBC units with soft-shell filters: filtration performance and impact on RBCs during storage for 42 days.Transfusion. 2002 Feb;42(2):153-8. doi: 10.1046/j.1537-2995.2002.00035.x. Transfusion. 2002. PMID: 11896328
-
Leukodepletion blood filters: filter design and mechanisms of leukocyte removal.Transfus Med Rev. 1993 Apr;7(2):65-77. doi: 10.1016/s0887-7963(93)70125-x. Transfus Med Rev. 1993. PMID: 8481601 Review.
References
-
- Gamonet C, Mourey G, Aupet S, Biichle S, Petitjean R, Vidal C, Pugin A, Naegelen C, Tiberghien P, Morel P, Angelot-Delettre F, Seilles E, Saas P, Bardiaux L, Garnache-Ottou F. How to quantify microparticles in RBCs? A validated flow cytometry method allows the detection of an increase in microparticles during storage. Transfusion. 2017. Mar;57(3):504-516. doi: 10.1111/trf.13989. - PubMed
-
- Maheen R, Yasir A, Omar N, Nazish M, Navida M, Rao SA, Muhammad R, Shahida M. Levels of red blood cells derived microparticles in stored erythrocyte concentrate. Afr J Pharm Pharmacol. 2020. Jul 31;14(6):185-91. doi: 10.5897/AJPP2020.5126
-
- Almizraq R, Tchir JD, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013. Oct;53(10):2258-67. doi: 10.1111/trf.12080. - PubMed
LinkOut - more resources
Full Text Sources

