Effect of Graded Sensorimotor Retraining on Pain Intensity in Patients With Chronic Low Back Pain: A Randomized Clinical Trial
- PMID: 35916848
- PMCID: PMC9346551
- DOI: 10.1001/jama.2022.9930
Effect of Graded Sensorimotor Retraining on Pain Intensity in Patients With Chronic Low Back Pain: A Randomized Clinical Trial
Abstract
Importance: The effects of altered neural processing, defined as altering neural networks responsible for perceptions of pain and function, on chronic pain remains unclear.
Objective: To estimate the effect of a graded sensorimotor retraining intervention (RESOLVE) on pain intensity in people with chronic low back pain.
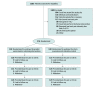
Design, setting, and participants: This parallel, 2-group, randomized clinical trial recruited participants with chronic (>3 months) nonspecific low back pain from primary care and community settings. A total of 276 adults were randomized (in a 1:1 ratio) to the intervention or sham procedure and attention control groups delivered by clinicians at a medical research institute in Sydney, Australia. The first participant was randomized on December 10, 2015, and the last was randomized on July 25, 2019. Follow-up was completed on February 3, 2020.
Interventions: Participants randomized to the intervention group (n = 138) were asked to participate in 12 weekly clinical sessions and home training designed to educate them about and assist them with movement and physical activity while experiencing lower back pain. Participants randomized to the control group (n = 138) were asked to participate in 12 weekly clinical sessions and home training that required similar time as the intervention but did not focus on education, movement, and physical activity. The control group included sham laser and shortwave diathermy applied to the back and sham noninvasive brain stimulation.
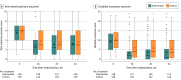
Main outcomes and measures: The primary outcome was pain intensity at 18 weeks, measured on an 11-point numerical rating scale (range, 0 [no pain] to 10 [worst pain imaginable]) for which the between-group minimum clinically important difference is 1.0 point.
Results: Among 276 randomized patients (mean [SD] age, 46 [14.3] years; 138 [50%] women), 261 (95%) completed follow-up at 18 weeks. The mean pain intensity was 5.6 at baseline and 3.1 at 18 weeks in the intervention group and 5.8 at baseline and 4.0 at 18 weeks in the control group, with an estimated between-group mean difference at 18 weeks of -1.0 point ([95% CI, -1.5 to -0.4]; P = .001), favoring the intervention group.
Conclusions and relevance: In this randomized clinical trial conducted at a single center among patients with chronic low back pain, graded sensorimotor retraining, compared with a sham procedure and attention control, significantly improved pain intensity at 18 weeks. The improvements in pain intensity were small, and further research is needed to understand the generalizability of the findings.
Trial registration: ANZCTR Identifier: ACTRN12615000610538.
Conflict of interest statement
Figures
Comment in
-
Graded Sensorimotor Retraining and Pain Intensity in Chronic Low Back Pain.JAMA. 2023 Jan 17;329(3):262. doi: 10.1001/jama.2022.21210. JAMA. 2023. PMID: 36648472 No abstract available.
References
-
- Abbafati C, Machado DB, Cislaghi B, et al. ; GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 - DOI - PMC - PubMed
-
- GBD results. Global Data Health Exchange . Accessed March 19, 2022. https://ghdx.healthdata.org/gbd-results-tool
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

