Repetitive Transcranial Magnetic Stimulation of the Brain After Ischemic Stroke: Mechanisms from Animal Models
- PMID: 35917043
- PMCID: PMC11412424
- DOI: 10.1007/s10571-022-01264-x
Repetitive Transcranial Magnetic Stimulation of the Brain After Ischemic Stroke: Mechanisms from Animal Models
Abstract
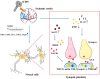
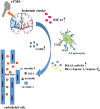
Stroke is a common cerebrovascular disease with high morbidity, mortality, and disability worldwide. Post-stroke dysfunction is related to the death of neurons and impairment of synaptic structure, which results from cerebral ischemic damage. Currently, transcranial magnetic stimulation (TMS) techniques are available to provide clinically effective interventions and quantitative diagnostic and prognostic biomarkers. The development of TMS has been 40 years and a range of repetitive TMS (rTMS) protocols are now available to regulate neuronal plasticity in many neurological disorders, such as stroke, Parkinson disease, psychiatric disorders, Alzheimer disease, and so on. Basic studies in an animal model with ischemic stroke are significant for demonstrating potential mechanisms of neural restoration induced by rTMS. In this review, the mechanisms were summarized, involving synaptic plasticity, neural cell death, neurogenesis, immune response, and blood-brain barrier (BBB) disruption in vitro and vivo experiments with ischemic stroke models. Those findings can contribute to the understanding of how rTMS modulated function recovery and the exploration of novel therapeutic targets. The mechanisms of rTMS in treating ischemic stroke from animal models. rTMS can prompt synaptic plasticity by increasing NMDAR, AMPAR and BDNF expression; rTMS can inhibit pro-inflammatory cytokines TNF and facilitate the expression of anti-inflammatory cytokines IL-10 by shifting astrocytic phenotypes from A1 to A2, and shifting microglial phenotypes from M1 to M2; rTMS facilitated the release of angiogenesis-related factors TGFβ and VEGF in A2 astrocytes, which can contribute to vasculogenesis and angiogenesis; rTMS can suppress apoptosis by increasing Bcl-2 expression and inhibiting Bax, caspase-3 expression; rTMS can also suppress pyroptosis by decreasing caspase-1, IL-1β, ASC, GSDMD and NLRP1 expression. rTMS, repetitive transcranial magnetic stimulation; NMDAR, N-methyl-D-aspartic acid receptors; AMPAR: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors; BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; GSDMD: cleaved Caspase-1 cleaves Gasdermin D; CBF: cerebral blood flow.
Keywords: Blood–brain barrier; Ischemic stroke; Neurogenesis; Synaptic plasticity; rTMS.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Theta-burst transcranial magnetic stimulation promotes stroke recovery by vascular protection and neovascularization.Theranostics. 2020 Oct 26;10(26):12090-12110. doi: 10.7150/thno.51573. eCollection 2020. Theranostics. 2020. PMID: 33204331 Free PMC article.
-
High-frequency repetitive transcranial magnetic stimulation ameliorates memory impairment by inhibiting neuroinflammation in the chronic cerebral hypoperfusion mice.Brain Behav. 2024 Jul;14(7):e3618. doi: 10.1002/brb3.3618. Brain Behav. 2024. PMID: 39010692 Free PMC article.
-
High-frequency repetitive transcranial magnetic stimulation improves functional recovery by inhibiting neurotoxic polarization of astrocytes in ischemic rats.J Neuroinflammation. 2020 May 6;17(1):150. doi: 10.1186/s12974-020-01747-y. J Neuroinflammation. 2020. PMID: 32375835 Free PMC article.
-
The neurobiological foundation of effective repetitive transcranial magnetic brain stimulation in Alzheimer's disease.Alzheimers Dement. 2025 Jun;21(6):e70337. doi: 10.1002/alz.70337. Alzheimers Dement. 2025. PMID: 40530618 Free PMC article. Review.
-
[Treatment of post stroke cognitive impairment by rTMS].Sheng Li Ke Xue Jin Zhan. 2012 Dec;43(6):411-6. Sheng Li Ke Xue Jin Zhan. 2012. PMID: 23520758 Review. Chinese.
Cited by
-
Amygdala-Targeted Relief of Neuropathic Pain: Efficacy of Repetitive Transcranial Magnetic Stimulation in NLRP3 Pathway Suppression.Mol Neurobiol. 2024 Nov;61(11):8904-8920. doi: 10.1007/s12035-024-04087-7. Epub 2024 Apr 4. Mol Neurobiol. 2024. PMID: 38573415 Free PMC article.
-
Interference with glutamate antiporter system xc - enables post-hypoxic long-term potentiation in hippocampus.Exp Physiol. 2024 Sep;109(9):1572-1592. doi: 10.1113/EP092045. Epub 2024 Aug 17. Exp Physiol. 2024. PMID: 39153228 Free PMC article.
-
Improving Upper Limb and Gait Rehabilitation Outcomes in Post-Stroke Patients: A Scoping Review on the Additional Effects of Non-Invasive Brain Stimulation When Combined with Robot-Aided Rehabilitation.Brain Sci. 2022 Nov 7;12(11):1511. doi: 10.3390/brainsci12111511. Brain Sci. 2022. PMID: 36358437 Free PMC article.
-
Combined Analysis of Human and Experimental Rat Samples Identified Biomarkers for Ischemic Stroke.Mol Neurobiol. 2025 Mar;62(3):3794-3812. doi: 10.1007/s12035-024-04512-x. Epub 2024 Sep 26. Mol Neurobiol. 2025. PMID: 39325100 Free PMC article.
-
Repetitive peripheral magnetic stimulation combined with transcranial magnetic stimulation in rehabilitation of upper extremity hemiparesis following stroke: a pilot study.J Rehabil Med. 2024 Feb 1;56:jrm19449. doi: 10.2340/jrm.v56.19449. J Rehabil Med. 2024. PMID: 38298134 Free PMC article. Clinical Trial.
References
-
- Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H et al (2009) Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 66(3):298–309. 10.1002/ana.21725 - PubMed
-
- Aras B, Inal O, Kesikburun S, Yasar E (2020) Response to speech and language therapy according to artery involvement and lesion location in post-stroke aphasia. J Stroke Cerebrovasc Dis 29(10):105132. 10.1016/j.jstrokecerebrovasdis.2020.105132 - PubMed
-
- Bolay H, Gursoy-Ozdemir Y, Unal I, Dalkara T (2000) Altered mechanisms of motor-evoked potential generation after transient focal cerebral ischemia in the rat: implications for transcranial magnetic stimulation. Brain Res 873(1):26–33. 10.1016/s0006-8993(00)02466-5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

