Visualizing Sphingosine-1-Phosphate Receptor 1(S1P1) Signaling During Central Nervous System De- and Remyelination
- PMID: 35917044
- PMCID: PMC10444542
- DOI: 10.1007/s10571-022-01245-0
Visualizing Sphingosine-1-Phosphate Receptor 1(S1P1) Signaling During Central Nervous System De- and Remyelination
Abstract
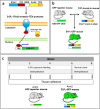
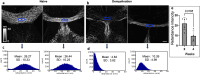
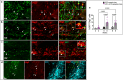
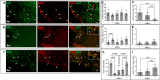
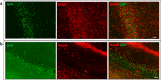
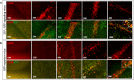
Multiple sclerosis (MS) is an inflammatory-demyelinating disease of the central nervous system (CNS) mediated by aberrant auto-reactive immune responses. The current immune-modulatory therapies are unable to protect and repair immune-mediated neural tissue damage. One of the therapeutic targets in MS is the sphingosine-1-phosphate (S1P) pathway which signals via sphingosine-1-phosphate receptors 1-5 (S1P1-5). S1P receptors are expressed predominantly on immune and CNS cells. Considering the potential neuroprotective properties of S1P signaling, we utilized S1P1-GFP (Green fluorescent protein) reporter mice in the cuprizone-induced demyelination model to investigate in vivo S1P - S1P1 signaling in the CNS. We observed S1P1 signaling in a subset of neural stem cells in the subventricular zone (SVZ) during demyelination. During remyelination, S1P1 signaling is expressed in oligodendrocyte progenitor cells in the SVZ and mature oligodendrocytes in the medial corpus callosum (MCC). In the cuprizone model, we did not observe S1P1 signaling in neurons and astrocytes. We also observed β-arrestin-dependent S1P1 signaling in lymphocytes during demyelination and CNS inflammation. Our findings reveal β-arrestin-dependent S1P1 signaling in oligodendrocyte lineage cells implying a role of S1P1 signaling in remyelination.
Keywords: Demyelination; GFP reporter mice; Multiple sclerosis; Oligodendrocyte; Sphingosine-1-phosphate receptor 1.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Deconstructing the Pharmacological Contribution of Sphingosine-1 Phosphate Receptors to Mouse Models of Multiple Sclerosis Using the Species Selectivity of Ozanimod, a Dual Modulator of Human Sphingosine 1-Phosphate Receptor Subtypes 1 and 5.J Pharmacol Exp Ther. 2021 Dec;379(3):386-399. doi: 10.1124/jpet.121.000741. Epub 2021 Sep 17. J Pharmacol Exp Ther. 2021. PMID: 34535564
-
Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum.Neuroimage. 2014 Feb 1;86:99-110. doi: 10.1016/j.neuroimage.2013.07.080. Epub 2013 Aug 7. Neuroimage. 2014. PMID: 23933305
-
Sphingosine kinase 2 is essential for remyelination following cuprizone intoxication.Glia. 2021 Dec;69(12):2863-2881. doi: 10.1002/glia.24074. Epub 2021 Aug 16. Glia. 2021. PMID: 34399014
-
Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy.J Neurol Sci. 2013 May 15;328(1-2):9-18. doi: 10.1016/j.jns.2013.02.011. Epub 2013 Mar 19. J Neurol Sci. 2013. PMID: 23518370 Free PMC article. Review.
-
How to Use the Cuprizone Model to Study De- and Remyelination.Int J Mol Sci. 2024 Jan 24;25(3):1445. doi: 10.3390/ijms25031445. Int J Mol Sci. 2024. PMID: 38338724 Free PMC article. Review.
Cited by
-
A Novel Mouse Model for Cerebral Inflammatory Demyelination in X-Linked Adrenoleukodystrophy: Insights into Pathogenesis and Potential Therapeutic Targets.Ann Neurol. 2025 Feb;97(2):296-312. doi: 10.1002/ana.27117. Epub 2024 Oct 28. Ann Neurol. 2025. PMID: 39467011
-
Sphingosine-1-phosphate signaling through Müller glia regulates neuroprotection and the accumulation of immune cells in the rodent retina.bioRxiv [Preprint]. 2025 Feb 6:2025.02.03.636254. doi: 10.1101/2025.02.03.636254. bioRxiv. 2025. PMID: 39975061 Free PMC article. Preprint.
-
A novel mouse model of cerebral adrenoleukodystrophy highlights NLRP3 activity in lesion pathogenesis.bioRxiv [Preprint]. 2023 Nov 10:2023.11.07.564025. doi: 10.1101/2023.11.07.564025. bioRxiv. 2023. PMID: 37986739 Free PMC article. Preprint.
References
-
- Anastasiadou S, Knoll B (2016) The multiple sclerosis drug fingolimod (FTY720) stimulates neuronal gene expression, axonal growth and regeneration. Exp Neurol 279:243–260. 10.1016/j.expneurol.2016.03.012 - PubMed
-
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK (2011) Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci USA 108(32):13287–13292. 10.1073/pnas.1107368108 - PMC - PubMed
-
- Baecher-Allan C, Kaskow BJ, Weiner HL (2018) Multiple sclerosis: mechanisms and immunotherapy. Neuron 97(4):742–768. 10.1016/j.neuron.2018.01.021 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

