Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis in the Russian Federation
- PMID: 35917057
- PMCID: PMC9464287
- DOI: 10.1007/s13555-022-00776-0
Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis in the Russian Federation
Abstract
Introduction: Risankizumab has demonstrated efficacy and safety in phase 3 studies in patients with moderate to severe plaque psoriasis. This randomized clinical trial assessed the efficacy and safety of risankizumab in patients with moderate to severe plaque psoriasis in the Russian Federation.
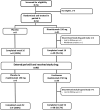
Methods: Patients with moderate to severe plaque psoriasis were randomized 4:1 to 16 weeks of double-blind treatment with risankizumab 150 mg or placebo (period A; dosing at baseline and week 4) followed by an open-label extension (period B) during which all patients received risankizumab 150 mg at weeks 16, 28, and 40 and were followed up to week 52. The primary study endpoint was the proportion of patients achieving ≥ 90% improvement in the Psoriasis Area and Severity Index (PASI 90) at week 16, and secondary endpoints included Static Physician's Global Assessment scores and the Dermatology Life Quality Index. Treatment-emergent adverse events were monitored throughout the two study periods.
Results: Of the 50 patients who entered period A, 41 were randomized to receive risankizumab and 9 to receive placebo. Forty-eight patients entered period B, and 47 completed the study. A significantly larger proportion of risankizumab-treated patients achieved PASI 90 at week 16 compared with placebo-treated patients [response rate difference: 38.8% (95% CI 7.8-69.7%; P = 0.035)]. Consistently higher proportions of risankizumab-treated patients achieved secondary endpoints compared with the placebo-treated patients. Safety profiles were similar between the treatment groups, and no patients discontinued the study drug owing to adverse events.
Conclusion: Risankizumab was efficacious and well tolerated in patients with moderate to severe plaque psoriasis in the Russian Federation.
Trial registration: ClinicalTrials.gov NCT03518047.
Keywords: Efficacy; Psoriasis; Risankizumab; Russia; Safety.
© 2022. The Author(s).
Figures



References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis Associated Comorbidity (IMPACT) Project Team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Investig Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. - DOI - PubMed
-
- World Health Organization. Global report on psoriasis. 2016. https://apps.who.int/iris/handle/10665/204417. Accessed 10 Feb 2021.
Associated data
LinkOut - more resources
Full Text Sources
Medical

