Lysosomal acid lipase, CSF1R, and PD-L1 determine functions of CD11c+ myeloid-derived suppressor cells
- PMID: 35917184
- PMCID: PMC9536279
- DOI: 10.1172/jci.insight.156623
Lysosomal acid lipase, CSF1R, and PD-L1 determine functions of CD11c+ myeloid-derived suppressor cells
Abstract
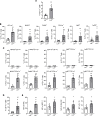
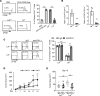
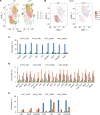
Lysosomal acid lipase (LAL) is a key enzyme in the metabolic pathway of neutral lipids. In the blood of LAL-deficient (Lal-/-) mice, increased CD11c+ cells were accompanied by upregulated programmed cell death ligand 1 (PD-L1) expression. Single-cell RNA sequencing of Lal-/- CD11c+ cells identified 2 distinctive clusters with a major metabolic shift toward glucose utilization and reactive oxygen species overproduction. Pharmacologically blocking pyruvate dehydrogenase in glycolysis not only reduced CD11c+ cells and their PD-L1 expression but also reversed their capabilities of T cell suppression and tumor growth stimulation. Colony-stimulating factor 1 receptor (CSF1R) played an essential role in controlling Lal-/- CD11c+ cell homeostasis and function and PD-L1 expression. Pharmacological inhibition of LAL activity increased CD11c, PD-L1, and CSF1R levels in both normal murine myeloid cells and human blood cells. Tumor-bearing mice and human patients with non-small cell lung cancer also showed CD11c+ cell expansion with PD-L1 and CSF1R upregulation and immunosuppression. There were positive correlations among CD11c, PD-L1, and CSF1R expression and negative correlations with LAL expression in patients with lung cancer or melanoma using The Cancer Genome Atlas database and patient samples. Therefore, CD11c+ cells switched their functions to immune suppression and tumor growth stimulation through CSF1R/PD-L1 upregulation and metabolic reprogramming.
Keywords: Cancer immunotherapy; Immunology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

