Glucose deprivation reduces proliferation and motility, and enhances the anti-proliferative effects of paclitaxel and doxorubicin in breast cell lines in vitro
- PMID: 35917304
- PMCID: PMC9345370
- DOI: 10.1371/journal.pone.0272449
Glucose deprivation reduces proliferation and motility, and enhances the anti-proliferative effects of paclitaxel and doxorubicin in breast cell lines in vitro
Abstract
Background: Breast cancer chemotherapy with high dose alkylating agents is severely limited by their collateral toxicity to crucial normal tissues such as immune and gut cells. Taking advantage of the selective dependence of cancer cells on high glucose and combining glucose deprivation with these agents could produce therapeutic synergy.
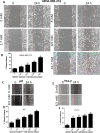
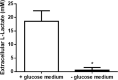
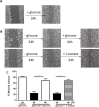
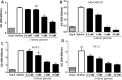
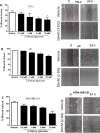
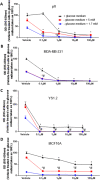
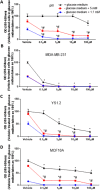
Methods: In this study we examined the effect of glucose as well as its deprivation, and antagonism using the non-metabolized analogue 2-deoxy glucose, on the proliferation of several breast cancer cell lines MCF7, MDA-MB-231, YS1.2 and pII and one normal breast cell line, using the MTT assay. Motility was quantitatively assessed using the wound healing assay. Lactate, as the end product of anaerobic glucose metabolism, secreted into culture medium was measured by a biochemical assay. The effect of paclitaxel and doxorubicin on cell proliferation was tested in the absence and presence of low concentrations of glucose using MTT assay.
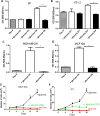
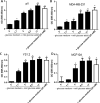
Results: In all cell lines, glucose supplementation enhanced while glucose deprivation reduced both their proliferation and motility. Lactate added to the medium could substitute for glucose. The inhibitory effects of paclitaxel and doxorubicin were significantly enhanced when glucose concentration was decreased in the culture medium, requiring 1000-fold lesser concentration to achieve a similar degree of inhibition to that seen in glucose-containing medium.
Conclusion: Our data show that a synergy was obtained by combining paclitaxel and doxorubicin with glucose reduction to inhibit cancer cell growth, which in vivo, might be achieved by applying a carbohydrate-restricted diet during the limited phase of application of chemotherapy; this could permit a dose reduction of the cytotoxic agents, resulting in greater tolerance and lesser side effects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Prolactin receptor antagonism reduces the clonogenic capacity of breast cancer cells and potentiates doxorubicin and paclitaxel cytotoxicity.Breast Cancer Res. 2008;10(4):R68. doi: 10.1186/bcr2129. Epub 2008 Aug 5. Breast Cancer Res. 2008. PMID: 18681966 Free PMC article.
-
Glycemic load impacts the response of acquired resistance in breast cancer cells to chemotherapeutic drugs in vitro.PLoS One. 2024 Nov 22;19(11):e0311345. doi: 10.1371/journal.pone.0311345. eCollection 2024. PLoS One. 2024. PMID: 39576770 Free PMC article.
-
Interactions of doxycycline with chemotherapeutic agents in human breast adenocarcinoma MDA-MB-231 cells.Anticancer Drugs. 2009 Feb;20(2):115-22. doi: 10.1097/CAD.0b013e32831c14ec. Anticancer Drugs. 2009. PMID: 19209028
-
Predicting tumour response to anti-HER1 therapy using medical imaging: a literature review and in vitro study of [18F]-FDG incorporation by breast cancer cells responding to cetuximab.Br J Biomed Sci. 2011;68(3):158-66. doi: 10.1080/09674845.2011.11730344. Br J Biomed Sci. 2011. PMID: 21950209 Review.
-
Current status of paclitaxel in the treatment of breast cancer.Breast Cancer Res Treat. 1995;33(1):27-37. doi: 10.1007/BF00666068. Breast Cancer Res Treat. 1995. PMID: 7749130 Review.
Cited by
-
Unveiling the cytotoxic and anti-proliferative potential of green-synthesized silver nanoparticles mediated by Colletotrichum gloeosporioides.RSC Adv. 2024 Jan 30;14(6):4074-4088. doi: 10.1039/d3ra06145k. eCollection 2024 Jan 23. RSC Adv. 2024. PMID: 38292267 Free PMC article.
-
Targeting fatty acid oxidation enhances response to HER2-targeted therapy.Nat Commun. 2024 Aug 3;15(1):6587. doi: 10.1038/s41467-024-50998-3. Nat Commun. 2024. PMID: 39097623 Free PMC article.
-
Mutation Status and Glucose Availability Affect the Response to Mitochondria-Targeted Quercetin Derivative in Breast Cancer Cells.Cancers (Basel). 2023 Nov 28;15(23):5614. doi: 10.3390/cancers15235614. Cancers (Basel). 2023. PMID: 38067318 Free PMC article.
-
The effect of GLP-1R agonists on the medical triad of obesity, diabetes, and cancer.Cancer Metastasis Rev. 2024 Dec;43(4):1297-1314. doi: 10.1007/s10555-024-10192-9. Epub 2024 May 27. Cancer Metastasis Rev. 2024. PMID: 38801466 Free PMC article. Review.
-
Pleiotropic Effects of Metformin on the Chemotherapy Response of HPV-Positive Cancer Cells.J Med Virol. 2025 Jun;97(6):e70434. doi: 10.1002/jmv.70434. J Med Virol. 2025. PMID: 40522309 Free PMC article.
References
-
- Liao S, Li J, Wei W, Wang L, Zhang Y, Li J, et al.. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prev. 2011;12(4):1061–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

