Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom
- PMID: 35917440
- PMCID: PMC9384604
- DOI: 10.1093/cid/ciac613
Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom
Abstract
Background: The SARS-CoV-2 Delta variant has been replaced by the highly transmissible Omicron BA.1 variant, and subsequently by Omicron BA.2. It is important to understand how these changes in dominant variants affect reported symptoms, while also accounting for symptoms arising from other co-circulating respiratory viruses.
Methods: In a nationally representative UK community study, the COVID-19 Infection Survey, we investigated symptoms in PCR-positive infection episodes vs. PCR-negative study visits over calendar time, by age and vaccination status, comparing periods when the Delta, Omicron BA.1 and BA.2 variants were dominant.
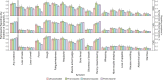
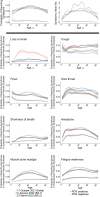
Results: Between October-2020 and April-2022, 120,995 SARS-CoV-2 PCR-positive episodes occurred in 115,886 participants, with 70,683 (58%) reporting symptoms. The comparator comprised 4,766,366 PCR-negative study visits (483,894 participants); 203,422 (4%) reporting symptoms. Symptom reporting in PCR-positives varied over time, with a marked reduction in loss of taste/smell as Omicron BA.1 dominated, maintained with BA.2 (44%/45% 17 October 2021, 16%/13% 2 January 2022, 15%/12% 27 March 2022). Cough, fever, shortness of breath, myalgia, fatigue/weakness and headache also decreased after Omicron BA.1 dominated, but sore throat increased, the latter to a greater degree than concurrent increases in PCR-negatives. Fatigue/weakness increased again after BA.2 dominated, although to a similar degree to concurrent increases in PCR-negatives. Symptoms were consistently more common in adults aged 18-65 years than in children or older adults.
Conclusions: Increases in sore throat (also common in the general community), and a marked reduction in loss of taste/smell, make Omicron harder to detect with symptom-based testing algorithms, with implications for institutional and national testing policies.
Keywords: Omicron; SARS-CoV-2; symptoms.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Figures


References
-
- Kozlov M. Omicron’s feeble attack on the lungs could make it less dangerous. 2022. Available at: https://www.nature.com/articles/d41586-022-00007-8. Accessed 10 January 2022. - PubMed
-
- Willett BJ, Grove J, Maclean OA, et al. The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. medRxiv [Preprint: not peer reviewed]. 26 January 2022. Available from: https://www.medrxiv.org/content/10.1101/2022.01.03.21268111v2. - DOI
-
- Meng B, Ferreira IATM, Abdullahi A, et al. SARS-CoV-2 Omicron spike mediated immune escape and tropism shift. bioRxiv [Preprint: not peer reviewed]. 22 December 2021. Available from: https://www.biorxiv.org/content/10.1101/2021.12.17.473248v2. - DOI
-
- Bentley EG, Kirby A, Sharma P, et al. SARS-CoV-2 Omicron-B.1.1.529 variant leads to less severe disease than Pango B and Delta variants strains in a mouse model of severe COVID-19. bioRxiv [Preprint: not peer reviewed]. 30 December 2021. Available from: https://www.biorxiv.org/content/10.1101/2021.12.26.474085v2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

