Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy
- PMID: 35917453
- PMCID: PMC10653009
- DOI: 10.1182/blood.2021014586
Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy
Abstract
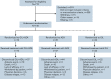
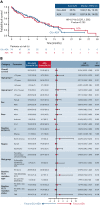
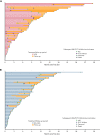
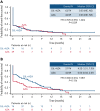
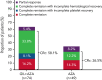
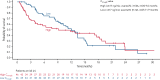
Treatment results for patients with newly diagnosed FMS-like tyrosine kinase 3 (FLT3)-mutated (FLT3mut+) acute myeloid leukemia (AML) ineligible for intensive chemotherapy are disappointing. This multicenter, open-label, phase 3 trial randomized (2:1) untreated adults with FLT3mut+ AML ineligible for intensive induction chemotherapy to receive gilteritinib (120 mg/d orally) and azacitidine (GIL + AZA) or azacitidine (AZA) alone. The primary end point was overall survival (OS). At the interim analysis (August 26, 2020), a total of 123 patients were randomized to treatment (GIL + AZA, n = 74; AZA, n = 49). Subsequent AML therapy, including FLT3 inhibitors, was received by 20.3% (GIL + AZA) and 44.9% (AZA) of patients. Median OS was 9.82 (GIL + AZA) and 8.87 (AZA) months (hazard ratio, 0.916; 95% CI, 0.529-1.585; P = .753). The study was closed based on the protocol-specified boundary for futility. Median event-free survival was 0.03 month in both arms. Event-free survival defined by using composite complete remission (CRc) was 4.53 months for GIL + AZA and 0.03 month for AZA (hazard ratio, 0.686; 95% CI, 0.433-1.087; P = .156). CRc rates were 58.1% (GIL + AZA) and 26.5% (AZA) (difference, 31.4%; 95% CI, 13.1-49.7; P < .001). Adverse event (AE) rates were similar for GIL + AZA (100%) and AZA (95.7%); grade ≥3 AEs were 95.9% and 89.4%, respectively. Common AEs with GIL + AZA included pyrexia (47.9%) and diarrhea (38.4%). Gilteritinib steady-state trough concentrations did not differ between GIL + AZA and gilteritinib. GIL + AZA resulted in significantly higher CRc rates, although similar OS compared with AZA. Results support the safety/tolerability and clinical activity of upfront therapy with GIL + AZA in older/unfit patients with FLT3mut+ AML. This trial was registered at www.clinicaltrials.gov as #NCT02752035.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.S.W. has received honoraria from Stemline, Kura, Pfizer, and DAVA Oncology; participated in advisory boards with AbbVie, Astellas, BMS/Celgene, Genentech, Gilead, GlaxoSmithKline, Jazz, Kite Pharmaceuticals, Kura Oncology, Novartis, Pfizer, Stemline, and Takeda; and participates in data monitoring committees for AbbVie and Rafael Pharmaceuticals. P.M. has participated in advisory boards and received honoraria from Astellas. M.D.M. has participated in advisory boards for Astellas, AbbVie, Trillium Therapeutics, Celgene, and Bristol Myers Squibb; and has received research funding from AbbVie. J.-H.L. has received honoraria from AbbVie Korea and Astellas Korea; and has participated in advisory boards for Astellas and AbbVie. M.H. has received honoraria from Jazz Pharmaceuticals, Janssen, and Novartis; participated in advisory boards for AbbVie, BMS/Celgene, Daiichi Sankyo, Jazz Pharmaceuticals, Novartis, Pfizer, Roche, and Tolremo; and has received research funding from Astellas, Bayer Pharma AG, BerGenBio, Daiichi Sankyo, Jazz Pharmaceuticals, Karyopharm, Novartis, Pfizer, and Roche. T.N. has received honoraria from Nippon Shinyaku, Bristol Myers Squibb, and Otsuka Pharma; and reports study funding from Astellas, Daiichi Sankyo, and Fujifilm. K.L. has received grants from Novartis, Takeda, Janssen, and AbbVie; and consulting fees from Novartis, Takeda, AbbVie, iQone, Astellas, Astra, and BeiGene. J.E. has received a grant from Novartis; honoraria from Astellas; and participated in advisory boards for Novartis, AbbVie, Jazz Pharmaceuticals, Pfizer, Celgene, and Daiichi Sankyo. J.K.A. has received advisory or consulting fees from AbbVie, Amgen, Astellas, bluebird bio, Curio Science, Daiichi Sankyo, Kura Oncology, Stemline, Syros, and Theradex; research funding at an institutional level for the conduct of trials from ALX Oncology, Amgen, Aptos, Astellas, Aprea, BioSight, BMS, Boehringer Ingelheim, Celgene, Fujifilm, ImmunoGen, Kartos, Kura Oncology, Loxo, and Takeda; reimbursement for travel from BioSight; and serves on a data monitoring committee for GlycoMimetics. V.H. has received honoraria fees from Novartis; travel support from Novartis and BMS; and participated in advisory boards for Incyte, Novartis, BMS, and AbbVie. E.P. has received consulting fees from KCR US, Inc.; honoraria from Amgen, Novartis, Servier, and Angelini Pharma; and travel support from Novartis, Servier, Angelini Pharma, Bristol Myers Squibb, Jazz Pharmaceuticals, Pfizer, and Astellas Pharma. J.E.H. reports holding stock for Ligacept, LLC. S.L., N.P., J.E.H., S.C.G., E.S.R., and R.V.T. are employees of Astellas. The remaining authors declare no competing financial interests.
The current affiliation for R.V.T. is Takeda Pharmaceutical Company, Boston, MA.
Figures

References
-
- Moreno I, Martín G, Bolufer P, et al. Incidence and prognostic value of FLT3internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88(1):19–24. - PubMed
-
- Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090–1098. - PubMed
-
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–4187. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

