[Pt(PPh3)4]-Catalyzed Selective Diboration of Symmetrical and Unsymmetrical 1,3-Diynes
- PMID: 35917577
- PMCID: PMC9396666
- DOI: 10.1021/acs.joc.2c00844
[Pt(PPh3)4]-Catalyzed Selective Diboration of Symmetrical and Unsymmetrical 1,3-Diynes
Abstract
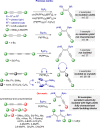
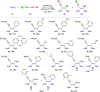
A straightforward, efficient, and selective method for the preparation of novel boryl-functionalized enynes or dienes via [Pt(PPh3)4]-catalyzed diboration of a broad spectrum of symmetrical and unsymmetrical 1,3-diynes was developed. The catalytic cycle of diboration was proposed on the basis of low-temperature 31P NMR studies. An alternative isolation method via product condensation on a cold finger was developed, which, in contrast to previous literature reports, eliminates the need for the additional transformation of rapidly decomposing enynyl pinacol boronates to more stable silica-based column chromatography derivatives during the separation step. To prove the efficiency of this simple catalytic protocol, bisboryl-functionalized enynes were synthesized in a gram scale and tested as useful building blocks in advanced organic transformations, such as hydrosilylation and Suzuki and sila-Sonogashira couplings. The presence of silyl, boryl, as well as other functions like halogen or alkoxy in their structures builds a new class of multifunctionalized enynes that might be modified in various chemical reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

