Emerging roles of ER-resident selenoproteins in brain physiology and physiopathology
- PMID: 35917681
- PMCID: PMC9344019
- DOI: 10.1016/j.redox.2022.102412
Emerging roles of ER-resident selenoproteins in brain physiology and physiopathology
Abstract
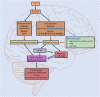
The brain has a very high oxygen consumption rate and is particularly sensitive to oxidative stress. It is also the last organ to suffer from a loss of selenium (Se) in case of deficiency. Se is a crucial trace element present in the form of selenocysteine, the 21st proteinogenic amino acid present in selenoproteins, an essential protein family in the brain that participates in redox signaling. Among the most abundant selenoproteins in the brain are glutathione peroxidase 4 (GPX4), which reduces lipid peroxides and prevents ferroptosis, and selenoproteins W, I, F, K, M, O and T. Remarkably, more than half of them are proteins present in the ER and recent studies have shown their involvement in the maintenance of ER homeostasis, glycoprotein folding and quality control, redox balance, ER stress response signaling pathways and Ca2+ homeostasis. However, their molecular functions remain mostly undetermined. The ER is a highly specialized organelle in neurons that maintains the physical continuity of axons over long distances through its continuous distribution from the cell body to the nerve terminals. Alteration of this continuity can lead to degeneration of distal axons and subsequent neuronal death. Elucidation of the function of ER-resident selenoproteins in neuronal pathophysiology may therefore become a new perspective for understanding the pathophysiology of neurological diseases. Here we summarize what is currently known about each of their molecular functions and their impact on the nervous system during development and stress.
Keywords: Brain pathologies; Calcium mobilization; ER protein Folding; Selenium; Thiol redox switch.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

