The quality of veterinary medicines and their implications for One Health
- PMID: 35918072
- PMCID: PMC9351321
- DOI: 10.1136/bmjgh-2022-008564
The quality of veterinary medicines and their implications for One Health
Abstract
Objective: Substandard and falsified (SF) veterinary medicines affect animal health, agricultural production and food security and will influence antimicrobial resistance (AMR) in both animals and humans. Yet, our understanding of their extent and impact is poor. We assess the available public domain evidence on the epidemiology of SF veterinary medicines, to better understand their prevalence and distribution and their public health impact on animals and humans.
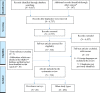
Methods: Searches were conducted in Embase, PubMed, MEDLINE, Global Health, Web of Science, CAB Abstracts, Scopus, Google Scholar, Google and websites with interest in veterinary medicines quality up to 28 February 2021. Identified articles in English and French were screened for eligibility. The Medicine Quality Assessment Reporting Guidelines were used to assess the quality of prevalence surveys.
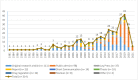
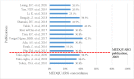
Results: Three hundred and fourteen publications were included with a failure frequency (the percentage of samples that failed at least one quality test) of 6.5% (2335/35 733). The majority of samples were from post-marketing surveillance by medicines regulatory authorities of the Republic of Korea and China. A small proportion (3.5%) of samples, all anti-infectives, were from 20 prevalence surveys, with more than half (53.1%, 662/1246) collected in low-income and lower middle-income countries in Africa and Asia. The prevalence survey sample size ranged from 4 to 310 samples (median (Q1-Q3): 50 (27-80)); 55.0% of surveys used convenience outlet sampling methods. In 20 prevalence surveys more than half of the samples (52.0%, 648/1246) failed at least one quality test. The most common defects reported were out-of-specification active pharmaceutical ingredient(s) (API) content, failure of uniformity of units and disintegration tests. Almost half of samples (49.7%, 239/481) that failed API content tests contained at least one of the stated APIs below pharmacopoeial limits. Fifty-two samples (4.2% of all samples) contained one or more incorrect API. One hundred and twenty-three publications described incidents (recalls/seizures/case reports) of SF veterinary medicines in 29 countries.
Conclusion: The data suggest that SF veterinary products are likely to be a serious animal and public health problem that has received limited attention. However, few studies of SF veterinary medicines are available and are geographically restricted. Lower API content and disintegration/dissolution than recommended by pharmacopoeial standards risks treatment failure, animal suffering and contribute to AMR. Our findings highlight the need of more research, with robust methodology, to better inform policy and implement measures to assure the quality of veterinary medicines within supply chains. The mechanism and impact of SF veterinary products on animal and human health, agricultural production, their economy and AMR need more transdisciplinary research.
Keywords: epidemiology; public health; systematic review.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: PN and CC are supported by the Wellcome Trust.
Figures
References
-
- 1 HealthforAnimals . Achieving the sustainable development goals: the value of healthier animals, 2021. Available: https://healthforanimals.org/downloads/library/achievingthesdgsvalueofhe... [Accessed 17 Jan 2022].
-
- World Organisation for Animal Health (OIE) . Animal health: a multifaceted challenges, 2015. Available: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Key_Documen... [Accessed 17 Jan 2022].
-
- O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. Rev. Antimicrob. Resist 2016. https://amr-review.org/sites/default/files/160518_Finalpaper_withcover.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
