Response to acute monotherapy for major depressive disorder in randomized, placebo controlled trials submitted to the US Food and Drug Administration: individual participant data analysis
- PMID: 35918097
- PMCID: PMC9344377
- DOI: 10.1136/bmj-2021-067606
Response to acute monotherapy for major depressive disorder in randomized, placebo controlled trials submitted to the US Food and Drug Administration: individual participant data analysis
Abstract
Objectives: To characterize individual participant level response distributions to acute monotherapy for major depressive disorder in randomized, placebo controlled trials submitted to the US Food and Drug Administration from 1979 to 2016.
Design: Individual participant data analysis.
Population: 232 randomized, double blind, placebo controlled trials of drug monotherapy for major depressive disorder submitted by drug developers to the FDA between 1979 and 2016, comprising 73 388 adult and child participants meeting the inclusion criteria for efficacy studies on antidepressants.
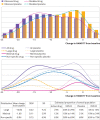
Main outcome measures: Responses were converted to Hamilton Rating Scale for Depression (HAMD17) equivalent scores where other measures were used to assess efficacy. Multivariable analyses examined the effects of age, sex, baseline severity, and year of the study on improvements in depressive symptoms in the antidepressant and placebo groups. Response distributions were analyzed with finite mixture models.
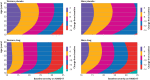
Results: The random effects mean difference between drug and placebo favored drug (1.75 points, 95% confidence interval 1.63 to 1.86). Differences between drug and placebo increased significantly (P<0.001) with greater baseline severity. After controlling for participant characteristics at baseline, no trends in treatment effect or placebo response over time were found. The best fitting model of response distributions was three normal distributions, with mean improvements from baseline to end of treatment of 16.0, 8.9, and 1.7 points. These distributions were designated Large, Non-specific, and Minimal responses, respectively. Participants who were treated with a drug were more likely to have a Large response (24.5% v 9.6%) and less likely to have a Minimal response (12.2.% v 21.5%).
Conclusions: The trimodal response distributions suggests that about 15% of participants have a substantial antidepressant effect beyond a placebo effect in clinical trials, highlighting the need for predictors of meaningful responses specific to drug treatment.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years except for BJM who reports serving as a member of the CMS Medicare Evidence Development and Coverage Advisory Committee and receiving fees outside the related work from the Federal Trade Commission, the Health Resources and Services Administration, the Heritage Foundation, and Oxidien Pharmaceuticals; BJM was a medical officer at the FDA in the Division of Psychiatry from 2016 to 2017; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- James SL, Abate D, Abate KH, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- Organization for Economic Cooperation and Development. Antidepressant drugs consumption, 2000 and 2015 (or nearest year). Health at a Glance 2017. OECD Indicators, OECD Publishing, Paris. 10.1787/health_glance-2017-graph181-en - DOI
-
- Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United States, 2011-2014. NCHS Data Brief 2017;(283):1-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
