Early Molecular Events Mediating Loss of Aquaporin-2 during Ureteral Obstruction in Rats
- PMID: 35918145
- PMCID: PMC9678028
- DOI: 10.1681/ASN.2022050601
Early Molecular Events Mediating Loss of Aquaporin-2 during Ureteral Obstruction in Rats
Abstract
Background: Ureteral obstruction is marked by disappearance of the vasopressin-dependent water channel aquaporin-2 (AQP2) in the renal collecting duct and polyuria upon reversal. Most studies of unilateral ureteral obstruction (UUO) models have examined late time points, obscuring the early signals that trigger loss of AQP2.
Methods: We performed RNA-Seq on microdissected rat cortical collecting ducts (CCDs) to identify early signaling pathways after establishment of UUO.
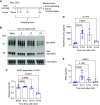
Results: Vasopressin V2 receptor (AVPR2) mRNA was decreased 3 hours after UUO, identifying one cause of AQP2 loss. Collecting duct principal cell differentiation markers were lost, including many not regulated by vasopressin. Immediate early genes in CCDs were widely induced 3 hours after UUO, including Myc, Atf3, and Fos (confirmed at the protein level). Simultaneously, expression of NF-κB signaling response genes known to repress Aqp2 increased. RNA-Seq for CCDs at an even earlier time point (30 minutes) showed widespread mRNA loss, indicating a "stunned" profile. Immunocytochemical labeling of markers of mRNA-degrading P-bodies DDX6 and 4E-T indicated an increase in P-body formation within 30 minutes.
Conclusions: Immediately after establishment of UUO, collecting ducts manifest a stunned state with broad disappearance of mRNAs. Within 3 hours, there is upregulation of immediate early and inflammatory genes and disappearance of the V2 vasopressin receptor, resulting in loss of AQP2 (confirmed by lipopolysaccharide administration). The inflammatory response seen rapidly after UUO establishment may be relevant to both UUO-induced polyuria and long-term development of fibrosis in UUO kidneys.
Keywords: P-body; RNA-Seq; aquaporin-2; collecting ducts; lipopolysaccharide; ureteral obstruction.
Copyright © 2022 by the American Society of Nephrology.
Figures









References
-
- Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: From molecules to medicine. Physiol Rev 82: 205–244, 2002 - PubMed
-
- Trepiccione F, Pisitkun T, Hoffert JD, Poulsen SB, Capasso G, Nielsen S, et al. : Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int 86: 757–767, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

