Targeting HECTD3-IKKα axis inhibits inflammation-related metastasis
- PMID: 35918322
- PMCID: PMC9345961
- DOI: 10.1038/s41392-022-01057-0
Targeting HECTD3-IKKα axis inhibits inflammation-related metastasis
Abstract
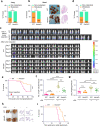
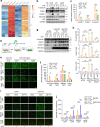
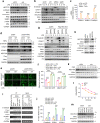
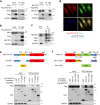
Metastasis is the leading cause of cancer-related death. The interactions between circulating tumor cells and endothelial adhesion molecules in distant organs is a key step during extravasation in hematogenous metastasis. Surgery is a common intervention for most primary solid tumors. However, surgical trauma-related systemic inflammation facilitates distant tumor metastasis by increasing the spread and adhesion of tumor cells to vascular endothelial cells (ECs). Currently, there are no effective interventions to prevent distant metastasis. Here, we show that HECTD3 deficiency in ECs significantly reduces tumor metastasis in multiple mouse models. HECTD3 depletion downregulates expression of adhesion molecules, such as VCAM-1, ICAM-1 and E-selectin, in mouse primary ECs and HUVECs stimulated by inflammatory factors and inhibits adhesion of tumor cells to ECs both in vitro and in vivo. We demonstrate that HECTD3 promotes stabilization, nuclear localization and kinase activity of IKKα by ubiquitinating IKKα with K27- and K63-linked polyubiquitin chains at K296, increasing phosphorylation of histone H3 to promote NF-κB target gene transcription. Knockout of HECTD3 in endothelium significantly inhibits tumor cells lung colonization, while conditional knockin promotes that. IKKα kinase inhibitors prevented LPS-induced pulmonary metastasis. These findings reveal the promotional role of the HECTD3-IKKα axis in tumor hematogenous metastasis and provide a potential strategy for tumor metastasis prevention.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Funaki S, et al. Novel approach for detection of isolated tumor cells in pulmonary vein using negative selection method: morphological classification and clinical implications. Eur. J. Cardiothorac. Surg. 2011;40:322–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

