Fundamental investigations on the sodium-ion transport properties of mixed polyanion solid-state battery electrolytes
- PMID: 35918385
- PMCID: PMC9345873
- DOI: 10.1038/s41467-022-32190-7
Fundamental investigations on the sodium-ion transport properties of mixed polyanion solid-state battery electrolytes
Erratum in
-
Author Correction: Fundamental investigations on the sodium-ion transport properties of mixed polyanion solid-state battery electrolytes.Nat Commun. 2022 Sep 21;13(1):5532. doi: 10.1038/s41467-022-33224-w. Nat Commun. 2022. PMID: 36131067 Free PMC article. No abstract available.
Abstract
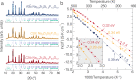
Lithium and sodium (Na) mixed polyanion solid electrolytes for all-solid-state batteries display some of the highest ionic conductivities reported to date. However, the effect of polyanion mixing on the ion-transport properties is still not fully understood. Here, we focus on Na1+xZr2SixP3-xO12 (0 ≤ x ≤ 3) NASICON electrolyte to elucidate the role of polyanion mixing on the Na-ion transport properties. Although NASICON is a widely investigated system, transport properties derived from experiments or theory vary by orders of magnitude. We use more than 2000 distinct ab initio-based kinetic Monte Carlo simulations to map the compositional space of NASICON over various time ranges, spatial resolutions and temperatures. Via electrochemical impedance spectroscopy measurements on samples with different sodium content, we find that the highest ionic conductivity (i.e., about 0.165 S cm-1 at 473 K) is experimentally achieved in Na3.4Zr2Si2.4P0.6O12, in line with simulations (i.e., about 0.170 S cm-1 at 473 K). The theoretical studies indicate that doped NASICON compounds (especially those with a silicon content x ≥ 2.4) can improve the Na-ion mobility compared to undoped NASICON compositions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Goodenough JB, Park K-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 2013;135:1167–1176. - PubMed
-
- Wang Y, et al. Design principles for solid-state lithium superionic conductors. Nat. Mater. 2015;14:1026–1031. - PubMed
-
- Bachman JC, et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 2016;116:140–162. - PubMed
-
- Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010;22:587–603.

