Continuous infusion versus intermittent infusion of vancomycin in critically ill patients undergoing continuous venovenous hemofiltration: a prospective interventional study
- PMID: 35918657
- PMCID: PMC9344630
- DOI: 10.1186/s12879-022-07618-6
Continuous infusion versus intermittent infusion of vancomycin in critically ill patients undergoing continuous venovenous hemofiltration: a prospective interventional study
Abstract
Background: A prospective interventional study comparing outcomes in critically ill patients receiving intermittent infusion (II) or continuous infusion (CI) of vancomycin during continuous venovenous hemofiltration (CVVH) is lacking. The objective of this study was to compare the pharmacokinetic/pharmacodynamics (PK/PD) target attainment, therapeutic efficacy and safety among critically ill patients who received CI or II of vancomycin in a prospective interventional trial and to explore the correlations of effluent flow rate (EFR) with PK/PD indices.
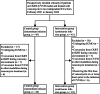
Methods: This prospective interventional study was conducted in two independent intensive care units (ICUs) from February 2021 to January 2022. Patients in one ICU were assigned to receive CI (intervention group) of vancomycin, whereas patients in the other ICU were assigned to receive II regimen (control group). The primary outcome was to compare the PK/PD target attainment, including target concentration and target area under the curve over 24 h to minimum inhibitory concentration (AUC24/MIC).
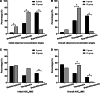
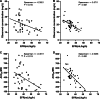
Results: Overall target attainment of PK/PD indices was higher with CI compared with II, irrespective of target concentration (78.7% vs. 40.5%; P < 0.05) or AUC24/MIC (53.2% vs. 28.6%; P < 0.05). There were no significant differences in clinical success (72.2% vs. 50.0%; P = 0.183) and microbiological success (83.3% vs. 75.0%, P = 0.681) between the patients treated with CI or II of vancomycin. Adverse reactions occurred at similar rates (0.0% vs. 4.4%; P = 0.462), and mortality between the two modalities was also not significant different (21.7% vs. 17.9%; P = 0.728). Correlation analysis showed a weak to moderately inverse correlation of EFR with observed concentration (r = - 0.3921, P = 0.01) and AUC24/MIC (r = - 0.3811, P = 0.013) in the II group, whereas the correlation between EFR and observed concentration (r = - 0.5711, P < 0.001) or AUC24/MIC (r = - 0.5458, P < 0.001) in the CI group was stronger.
Conclusion: As compared to II, CI of vancomycin in critically ill patients undergoing CVVH was associated with improved attainment of PK/PD indices. Furthermore, the inverse correlation of PK/PD indices with EFR was stronger among patients treated with CI of vancomycin. Trial registration The trial was registered in the Chinese clinical trial registration center (21/01/2021-No. ChiCTR2100042393).
Keywords: Continuous infusion; Continuous venovenous hemofiltration; Intermittent infusion; Prospective interventional study; Vancomycin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Prospective evaluation of a continuous infusion vancomycin dosing nomogram in critically ill patients undergoing continuous venovenous haemofiltration.J Antimicrob Chemother. 2018 Jan 1;73(1):199-203. doi: 10.1093/jac/dkx356. J Antimicrob Chemother. 2018. PMID: 29040561
-
Pharmacokinetics of piperacillin in critically ill patients receiving continuous venovenous haemofiltration: A randomised controlled trial of continuous infusion versus intermittent bolus administration.Int J Antimicrob Agents. 2015 Jul;46(1):39-44. doi: 10.1016/j.ijantimicag.2015.02.014. Epub 2015 Mar 28. Int J Antimicrob Agents. 2015. PMID: 25881872 Clinical Trial.
-
Pharmacokinetics and Pharmacodynamics of Extended-Infusion Cefepime in Critically Ill Patients Receiving Continuous Renal Replacement Therapy: A Prospective, Open-Label Study.Pharmacotherapy. 2019 Nov;39(11):1066-1076. doi: 10.1002/phar.2332. Epub 2019 Oct 22. Pharmacotherapy. 2019. PMID: 31549737
-
Optimal Meropenem Dosing Regimens in Patients Undergoing Continuous Renal Replacement Therapy: Systematic Review and Monte Carlo Simulations.Blood Purif. 2023;52(6):503-515. doi: 10.1159/000529694. Epub 2023 May 5. Blood Purif. 2023. PMID: 37231811
-
Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: A Systematic Review and Meta-Analysis.Crit Care Med. 2020 Jun;48(6):912-918. doi: 10.1097/CCM.0000000000004326. Crit Care Med. 2020. PMID: 32317590
Cited by
-
Antibiotic Therapy in the Critically Ill with Acute Renal Failure and Renal Replacement Therapy: A Narrative Review.Antibiotics (Basel). 2022 Dec 7;11(12):1769. doi: 10.3390/antibiotics11121769. Antibiotics (Basel). 2022. PMID: 36551426 Free PMC article. Review.
-
Population Pharmacokinetic/Pharmacodynamic Study of Linezolid in Hospital-Acquired Pneumonia Patients with Renal Insufficiency.Drug Des Devel Ther. 2024 Nov 8;18:5073-5086. doi: 10.2147/DDDT.S474470. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39545249 Free PMC article.
References
-
- Turner RB, Kojiro K, Shephard EA, Won R, Chang E, Chan D, Elbarbry F. Review and validation of Bayesian dose-optimizing software and equations for calculation of the vancomycin area under the curve in critically ill patients. Pharmacotherapy. 2018;38(12):1174–1183. doi: 10.1002/phar.2191. - DOI - PubMed
-
- Roberts JA, Joynt GM, Lee A, Choi G, Bellomo R, Kanji S, et al. The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: data from the multinational sampling antibiotics in renal replacement therapy study. Clin Infect Dis. 2021;72(8):1369–1378. doi: 10.1093/cid/ciaa224. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- SYSD2018243/the Suzhou Science and Technology Project
- SYSD2019189/the Suzhou Science and Technology Project
- SKJYD2021170/the Suzhou Science and Technology Project
- SKJYD2021171/the Suzhou Science and Technology Project
- SKJYD2021172/the Suzhou Science and Technology Project
- No. 320.6750.2020-12-69/Wu Jieping Medical Foundation
- No. A201816/Jiangsu Pharmaceutical Association. Hospital Pharmacy Research Project
- A201914/Jiangsu Pharmaceutical Association. Hospital Pharmacy Research Project
- A201915/Jiangsu Pharmaceutical Association. Hospital Pharmacy Research Project
- H202109/Jiangsu Pharmaceutical Association. Hospital Pharmacy Research Project
- No. LCZX202112/Suzhou special technical project for diagnosis and treatment of key clinical diseases
- No. KJXW2018024/Suzhou Ke Jiao Xing Wei Youth Science and Technology Projects
- No. Syhky201805/Suzhou Pharmaceutical Association. Hospital Pharmacy Research Project
LinkOut - more resources
Full Text Sources
Research Materials

